Abstract
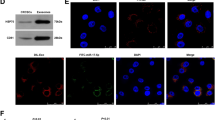
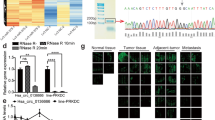
Recent studies have shown that tumor-derived exosomes participate in the communication between tumor cells and their microenvironment and mediate malignant biological behaviors including immune escape. In this study, we found that gastric cancer (GC) cell-derived exosomes could be effectively uptaken by Vγ9Vδ2 T cells, decrease the cell viability of Vγ9Vδ2 T cells, induce apoptosis, and reduce the production of cytotoxic cytokines IFN-γ and TNF-α. Furthermore, we demonstrated that exosomal miR-135b-5p was delivered into Vγ9Vδ2 T cells. Exosomal miR-135b-5p impaired the function of Vγ9Vδ2 T cells by targeting specificity protein 1 (SP1). More importantly, blocking the SP1 function by Plicamycin, an SP1 inhibitor, abolished the effect of stable miR-135b-5p knockdown GC cell-derived exosomes on Vγ9Vδ2 T cell function. Collectively, our results suggest that GC cell-derived exosomes impair the function of Vγ9Vδ2 T cells via miR-135b-5p/SP1 pathway, and targeting exosomal miR-135b-5p/SP1 axis may improve the efficiency of GC immunotherapy based on Vγ9Vδ2 T cells.







Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (6):394–424. doi:https://doi.org/10.3322/caac.21492
Feng R, Zong Y, Cao S, Xu R (2019) Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer communications (London, England) 39(1):22. https://doi.org/10.1186/s40880-019-0368-6
Coutzac C, Pernot S, Chaput N, Zaanan A (2019) Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol 133:25–32. https://doi.org/10.1016/j.critrevonc.2018.10.007
Olino K, Park T, Ahuja N (2020) Exposing hidden targets: combining epigenetic and immunotherapy to overcome cancer resistance. Semin Cancer Biol 65:114–122. https://doi.org/10.1016/j.semcancer.2020.01.001
Matsueda S, Graham D (2014) Immunotherapy in gastric cancer. World J Gastroenterol 20(7):1657–1666. https://doi.org/10.3748/wjg.v20.i7.1657
Kim T, da Silva E, Coit D, Tang L (2019) Intratumoral immune response to gastric cancer varies by molecular and histologic subtype. Am J Surg Pathol 43(6):851–860. https://doi.org/10.1097/pas.0000000000001253
Jones J, Smyth E (2020) Gastroesophageal cancer: Navigating the immune and genetic terrain to improve clinical outcomes. Cancer Treat Rev 84:101950. https://doi.org/10.1016/j.ctrv.2019.101950
Girardi M (2006) Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol 126(1):25–31. https://doi.org/10.1038/sj.jid.5700003
Gentles A, Newman A, Liu C, Bratman S, Feng W, Kim D, Nair V, Xu Y, Khuong A, Hoang C, Diehn M, West R, Plevritis S, Alizadeh A (2015) The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21(8):938–945. https://doi.org/10.1038/nm.3909
Fisher J, Anderson J (2018) Engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol 9:1409. https://doi.org/10.3389/fimmu.2018.01409
Joalland N, Lafrance L, Oullier T, Marionneau-Lambot S, Loussouarn D, Jarry U, Scotet E (2019) in vivoCombined chemotherapy and allogeneic human Vγ9Vδ2 T lymphocyte-immunotherapies efficiently control the development of human epithelial ovarian cancer cells. Oncoimmunology 8(11):e1649971. https://doi.org/10.1080/2162402x.2019.1649971
Zysk A, DeNichilo M, Panagopoulos V, Zinonos I, Liapis V, Hay S, Ingman W, Ponomarev V, Atkins G, Findlay D, Zannettino A, Evdokiou A (2017) Adoptive transfer of ex vivo expanded Vγ9Vδ2 T cells in combination with zoledronic acid inhibits cancer growth and limits osteolysis in a murine model of osteolytic breast cancer. Cancer Lett 386:141–150. https://doi.org/10.1016/j.canlet.2016.11.013
Wada I, Matsushita H, Noji S, Mori K, Yamashita H, Nomura S, Shimizu N, Seto Y, Kakimi K (2014) Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med 3(2):362–375. https://doi.org/10.1002/cam4.196
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Qu J, Qu X, Zhao M, Teng Y, Zhang Y, Hou K, Jiang Y, Yang X, Liu Y (2009) Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 41(12):875–880. https://doi.org/10.1016/j.dld.2009.04.006
Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, Wang Z, Chen L (2016) CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 19(3):754–766. https://doi.org/10.1007/s10120-015-0523-y
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W (2015) Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell cycle (Georgetown, Tex) 14(15):2473–2483. https://doi.org/10.1080/15384101.2015.1005530
Wang F, Li B, Wei Y, Zhao Y, Wang L, Zhang P, Yang J, He W, Chen H, Jiao Z, Li Y (2018) Tumor-derived exosomes induce PD1 macrophage population in human gastric cancer that promotes disease progression. Oncogenesis 7(5):41. https://doi.org/10.1038/s41389-018-0049-3
Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, Rong H, Wang W, Zhang D, Zhang Z, Tu S, Zhu X, Zhang Q (2019) Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer management and research 11:4023–4040. https://doi.org/10.2147/cmar.S198886
Ni C, Fang Q, Chen W, Jiang J, Jiang Z, Ye J, Zhang T, Yang L, Meng F, Xia W, Zhong M, Huang J (2020) Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+γδ1 Treg cells. Signal Transduct Target Ther 5(1):41. https://doi.org/10.1038/s41392-020-0129-7
Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, Wang S, Jiang J, Lang J, Zhu G (2019) Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene 38(15):2830–2843. https://doi.org/10.1038/s41388-018-0627-z
Feng C, She J, Chen X, Zhang Q, Zhang X, Wang Y, Ye J, Shi J, Tao J, Feng M, Guan W, Xia H, Zhang W, Xu G (2019) Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine (Lond) 14(19):2579–2593. https://doi.org/10.2217/nnm-2019-0053
O’Connell R, Rao D, Chaudhuri A, Baltimore D (2010) Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10(2):111–122. https://doi.org/10.1038/nri2708
Xie Y, Dang W, Zhang S, Yue W, Yang L, Zhai X, Yan Q, Lu J (2019) The role of exosomal noncoding RNAs in cancer. Mol Cancer 18(1):37. https://doi.org/10.1186/s12943-019-0984-4
Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K (2020) The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol 13(1):25. https://doi.org/10.1186/s13045-020-00848-8
Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, Ji R, Xu W, Qian H, Zhang X (2020) Extracellular vesicles from gastric cancer cells induce pd-l1 expression on neutrophils to suppress T-cell Immunity. Front Oncol 10:629. https://doi.org/10.3389/fonc.2020.00629
Chen C, Luo F, Liu X, Lu L, Xu H, Yang Q, Xue J, Shi L, Li J, Zhang A, Liu Q (2017) NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett 388:21–33. https://doi.org/10.1016/j.canlet.2016.11.027
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285(23):17442–17452. https://doi.org/10.1074/jbc.M110.107821
Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W (2018) Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 17(1):147. https://doi.org/10.1186/s12943-018-0897-7
Chen Z, Gao Y, Gao S, Song D, Feng Y (2020) MiR-135b-5p promotes viability, proliferation, migration and invasion of gastric cancer cells by targeting Krüppel-like factor 4 (KLF4). Archives of medical science : AMS 16(1):167–176. https://doi.org/10.5114/aoms.2019.87761
Zhang X, Mao H, Zhang S, Sun L, Zhang W, Chen Q, Wang L, Liu H (2020) lncRNA PCAT18 inhibits proliferation, migration and invasion of gastric cancer cells through miR-135b suppression to promote CLDN11 expression. Life Sci 249:117478. https://doi.org/10.1016/j.lfs.2020.117478
Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y (2019) miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Molecular therapy : the journal of the American Society of Gene Therapy 27(10):1772–1783. https://doi.org/10.1016/j.ymthe.2019.06.018
Cheng H, Chen L, Hu X, Qiu H, Xu X, Gao L, Tang G, Zhang W, Wang J, Yang J, Huang C (2019) Knockdown of MAML1 inhibits proliferation and induces apoptosis of T-cell acute lymphoblastic leukemia cells through SP1-dependent inactivation of TRIM59. J Cell Physiol 234(4):5186–5195. https://doi.org/10.1002/jcp.27323
Yao Y, Hu J, Shen Z, Yao R, Liu S, Li Y, Cong H, Wang X, Qiu W, Yue L (2015) MiR-200b expression in breast cancer: a prognostic marker and act on cell proliferation and apoptosis by targeting Sp1. J Cell Mol Med 19(4):760–769. https://doi.org/10.1111/jcmm.12432
Koyama D, Kikuchi J, Hiraoka N, Wada T, Kurosawa H, Chiba S, Furukawa Y (2014) Proteasome inhibitors exert cytotoxicity and increase chemosensitivity via transcriptional repression of Notch1 in T-cell acute lymphoblastic leukemia. Leukemia 28(6):1216–1226. https://doi.org/10.1038/leu.2013.366
Yu J, Wei M, Boyd Z, Lehmann E, Trotta R, Mao H, Liu S, Becknell B, Jaung M, Jarjoura D, Marcucci G, Wu L, Caligiuri M (2007) Transcriptional control of human T-BET expression: the role of Sp1. Eur J Immunol 37(9):2549–2561. https://doi.org/10.1002/eji.200737088
Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, Zhang G, Chen W (2020) B7–H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology 9(1):1748991. https://doi.org/10.1080/2162402x.2020.1748991
Fisher J, Heuijerjans J, Yan M, Gustafsson K, Anderson J (2014) γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 3(1):e27572. https://doi.org/10.4161/onci.27572
Lafont V, Sanchez F, Laprevotte E, Michaud H, Gros L, Eliaou J, Bonnefoy N (2014) Plasticity of γδ T Cells: Impact on the Anti-Tumor Response. Front Immunol 5:622. https://doi.org/10.3389/fimmu.2014.00622
Hoeres T, Smetak M, Pretscher D, Wilhelm M (2018) Improving the efficiency of Vγ9Vδ2 T-cell immunotherapy in cancer. Front Immunol 9:800. https://doi.org/10.3389/fimmu.2018.00800
Nicol A, Tokuyama H, Mattarollo S, Hagi T, Suzuki K, Yokokawa K, Nieda M (2011) Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 105(6):778–786. https://doi.org/10.1038/bjc.2011.293
Noguchi A, Kaneko T, Kamigaki T, Fujimoto K, Ozawa M, Saito M, Ariyoshi N, Goto S (2011) Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 13(1):92–97. https://doi.org/10.3109/14653249.2010.515581
Zhao Y, Niu C, Cui J (2018) Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med 16(1):3. https://doi.org/10.1186/s12967-017-1378-2
Maybruck B, Pfannenstiel L, Diaz-Montero M, Gastman B (2017) Tumor-derived exosomes induce CD8 T cell suppressors. J Immunother Cancer 5(1):65. https://doi.org/10.1186/s40425-017-0269-7
Vignard V, Labbé M, Marec N, André-Grégoire G, Jouand N, Fonteneau J, Labarrière N, Fradin D (2020) MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunol Res 8(2):255–267. https://doi.org/10.1158/2326-6066.Cir-19-0522
Liu F, Bu Z, Zhao F, Xiao D (2018) Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci 109(1):65–73. https://doi.org/10.1111/cas.13429
Whiteside T (2016) Exosomes and tumor-mediated immune suppression. J Clin Investig 126(4):1216–1223. https://doi.org/10.1172/jci81136
Huang Y, Liu K, Li Q, Yao Y, Wang Y (2018) Exosomes function in tumor immune microenvironment. Adv Exp Med Biol 1056:109–122. https://doi.org/10.1007/978-3-319-74470-4_7
Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z (2018) Hypoxic tumor-derived exosomal mir-301a mediates m2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Can Res 78(16):4586–4598. https://doi.org/10.1158/0008-5472.Can-17-3841
Mrizak D, Martin N, Barjon C, Jimenez-Pailhes A, Mustapha R, Niki T, Guigay J, Pancré V, de Launoit Y, Busson P, Moralès O, Delhem N (2015) Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst 107(1):363. https://doi.org/10.1093/jnci/dju363
Wu Y, Hu G, Wu R, Gong N (2020) High expression of miR-135b predicts malignant transformation and poor prognosis of gastric cancer. Life Sci 257:118133. https://doi.org/10.1016/j.lfs.2020.118133
Han T, Voon D, Oshima H, Nakayama M, Echizen K, Sakai E, Yong Z, Murakami K, Yu L, Minamoto T, Ock C, Jenkins B, Kim S, Yang H, Oshima M (2019) Interleukin 1 Up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology 156(4):1140-1155.e1144. https://doi.org/10.1053/j.gastro.2018.11.059
Shao L, Chen Z, Soutto M, Zhu S, Lu H, Romero-Gallo J, Peek R, Zhang S, El-Rifai W (2019) Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33(1):264–274. https://doi.org/10.1096/fj.201701456RR
Huang J, Shen M, Yan M, Cui Y, Gao Z, Meng X (2019) Exosome-mediated transfer of miR-1290 promotes cell proliferation and invasion in gastric cancer via NKD1. Acta Biochim Biophys Sin 51(9):900–907. https://doi.org/10.1093/abbs/gmz077
Li Q, Li B, Li Q, Wei S, He Z, Huang X, Wang L, Xia Y, Xu Z, Li Z, Wang W, Yang L, Zhang D, Xu Z (2018) Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis 9(9):854. https://doi.org/10.1038/s41419-018-0928-8
Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, Wang M, Sun Z, Qian H, Xu W (2018) Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol Carcinog 57(9):1223–1236. https://doi.org/10.1002/mc.22838
Liu X, Lu Y, Xu Y, Hou S, Huang J, Wang B, Zhao J, Xia S, Fan S, Yu X, Du Y, Hou L, Li Z, Ding Z, An S, Huang B, Li L, Tang J, Ju J, Guan H, Song B (2019) Exosomal transfer of miR-501 confers doxorubicin resistance and tumorigenesis via targeting of BLID in gastric cancer. Cancer Lett 459:122–134. https://doi.org/10.1016/j.canlet.2019.05.035
Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, Li S, Sun W, Deng T, Zhang L, Ying G, Ba Y (2018) Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting c-myb in vascular endothelial cells. Molecular therapy : the journal of the American Society of Gene Therapy 26(10):2466–2475. https://doi.org/10.1016/j.ymthe.2018.07.023
Zhou Z, Zhang H, Deng T, Ning T, Liu R, Liu D, Bai M, Ying G, Ba Y (2019) Exosomes carrying MicroRNA-155 target forkhead box O3 of endothelial cells and promote angiogenesis in gastric cancer. Molecular therapy oncolytics 15:223–233. https://doi.org/10.1016/j.omto.2019.10.006
Black A, Black J, Azizkhan-Clifford J (2001) Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol 188(2):143–160. https://doi.org/10.1002/jcp.1111
Wang R, Xu B, Xu H (2018) TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell cycle (Georgetown, Tex) 17 (24) doi:https://doi.org/10.1080/15384101.2018.1556063
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81802843, 82073156, 81672372); Suzhou Science & Technology plan project (SYS2019035, SS2019077).
Author information
Authors and Affiliations
Contributions
JL, TS, and WC designed the experiments; JL, LS, YC, and JZ performed most of the experiments; JS, JW, and GZ assisted with experiments and analysis of the data; MW and YG contributed to provide clinical samples; JL, TS, and WC wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Sun, L., Chen, Y. et al. Gastric cancer-derived exosomal miR-135b-5p impairs the function of Vγ9Vδ2 T cells by targeting specificity protein 1. Cancer Immunol Immunother 71, 311–325 (2022). https://doi.org/10.1007/s00262-021-02991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02991-8