Abstract
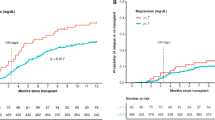
For many decades, selenium (Se) has been known as a potential anti-cancer agent that can also improve the function of immune cells in a variety of solid tumors. However, there is no report on the role of Se on CD4+ T cell subsets like CD4+CD25+FOXP3+ regulatory T cells (Tregs) in lymphoma patients. In this randomized clinical trial, we investigated the effect of 3-month Se consumption on the frequency of CD4+CD25+FOXP3+ Tregs and the expression of immune checkpoint receptors in thirty-two non-Hodgkin lymphoma (NHL) patients (16 patients with Se (Se+) and 16 without Se (Se−) consumption) with diffuse large B-cell lymphoma (DLBCL) subtype at stable remission. The change in the frequency of Tregs and expression of immune checkpoint receptors including CTLA-4, LAG-3, TIM-3, and PD-L1 genes were evaluated after 3 months in both groups using flow cytometry and SYBR Green Real-time PCR method, respectively. The results showed that the frequency of CD4+CD25+FOXP3+ Tregs and expression of immune checkpoint receptors did not significantly change after 3-month Se consumption in DLBCL patients. However, alteration in the frequency of CD4+CD25−FOXP3+ Treg subsets was positively correlated with change in CTLA-4, LAG-3, and TIM-3 expression in the Se+ group. Three-month Se supplementation did not prevent relapse in Se+ group. Taken together, Se supplementation alone did not affect the frequency of CD4+CD25+FOXP3+ Tregs, expression of checkpoint receptors, and prevention of relapse in DLBCL patients at stable remission phase but might influence the functional properties of other Treg subsets like CD4+CD25−FOXP3+ Tregs.




Similar content being viewed by others
References
Shankland KR, Armitage JO, Hancock BW (2012) Non-hodgkin lymphoma. Lancet 380(9844):848–857
Ansell SM (2017) Harnessing the power of the immune system in non-Hodgkin lymphoma: immunomodulators, checkpoint inhibitors, and beyond. Hematol 2014 Am Soc Hematol Educ Prog Book 2017(1):618–21
D’Arena G, Vitale C, Coscia M, Festa A, Di Minno NMD, De Feo V et al (2017) Regulatory T Cells and their prognostic relevance in hematologic malignancies. J Immunol Res 2017:1832968
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24(34):5373–5380
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T et al (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13(3):902–911
Dehghani M, Kalani M, Golmoghaddam H, Ramzi M, Arandi N (2020) Aberrant peripheral blood CD4 (+) CD25 (+) FOXP3 (+) regulatory T cells/T helper-17 number is associated with the outcome of patients with lymphoma. Cancer Immunol Immunother 69(9):1917–1928
Głowala-Kosińska M, Chwieduk A, Nieckula J, Saduś-Wojciechowska M, Grosicki S, Rusin A et al (2013) Association of circulating regulatory T cell number with the incidence and prognosis of diffuse large B-cell lymphoma. Eur J Haematol 91(2):122–128
Cha Z, Gu H, Zang Y, Wang Z, Li J, Huang W et al (2018) The prevalence and function of CD4(+)CXCR5(+)Foxp3(+) follicular regulatory T cells in diffuse large B cell lymphoma. Int Immunopharmacol 61:132–139
Nirschl CJ, Drake CG (2013) Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 19(18):4917–4924
Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39
La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB (2015) Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacother J Hum Pharmacol Drug Ther 35(10):963–76
Evans SO, Khairuddin PF, Jameson MB (2017) Optimising selenium for modulation of cancer treatments. Anticancer Res 37(12):6497–6509
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268
Wallenberg M, Misra S, Björnstedt M (2014) Selenium cytotoxicity in cancer. Basic Clin Pharmacol Toxicol 114(5):377–386
Davis CD (2012) Selenium supplementation and cancer prevention. Curr Nutr Rep 1(1):16–23
Gandin V, Khalkar P, Braude J, Fernandes AP (2018) Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic Biol Med 127:80–97
Asfour IA, El Shazly S, Fayek MH, Hegab HM, Raouf S, Moussa MA (2006) Effect of high-dose sodium selenite therapy on polymorphonuclear leukocyte apoptosis in non-Hodgkin’s lymphoma patients. Biol Trace Elem Res 110(1):19–32
Asfour IA, El-Tehewi MM, Ahmed MH, Abdel-Sattar MA, Moustafa NN, Hegab HM et al (2009) High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin’s lymphoma. Biol Trace Elem Res 127(3):200
Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H et al (2007) The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: correlation with response and survival. Biol Trace Elem Res 120(1–3):1–10
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients 10(9):1203
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):705–743
MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C et al (2010) Acute selenium toxicity associated with a dietary supplement. Arch Intern Med 170(3):256–261
Stevens J, Waters R, Sieniawska C, Kassam S, Montoto S, Fitzgibbon J et al (2011) Serum selenium concentration at diagnosis and outcome in patients with haematological malignancies. Br J Haematol 154(4):448–456
Last KW, Cornelius V, Delves T, Sieniawska C, Fitzgibbon J, Norton A et al (2003) Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J Clin Oncol 21(12):2335–2341
Sang L-X, Chang B, Zhu J-F, Yang F-L, Li Y, Jiang X-F et al (2017) Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4 (+) CD25 (+) regulatory T-cell responses. World J Gastroenterol 23(21):3850
Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X et al (2010) Selenium upregulates CD4+ CD25+ regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD. H-2h4 mice. Endocr J 57(7):595–601
Lobb RJ, Jacobson GM, Cursons RT, Jameson MB (2018) The interaction of selenium with chemotherapy and radiation on normal and malignant human mononuclear blood cells. Int J Mol Sci 19(10):3167
Nair D, Rådestad E, Khalkar P, Diaz-Argelich N, Schröder A, Klynning C et al (2018) Methylseleninic acid sensitizes ovarian cancer cells to T-cell mediated killing by decreasing PDL1 and VEGF levels. Front Oncol 8:407
Acknowledgements
This study was funded by a grant provided by Shiraz University of Medical Sciences (Grant Number 93-01-01-8106), approved by the Clinical Trial Registry of Shiraz University of Medical Sciences (RCT Number: IR.SUMS.REC.1396.S210) and also registered in the Iranian Registry of Clinical Trials (IRCT ID: IRCT20200128046292N1).
Funding
This study was financially supported by a grant provided by Shiraz University of Medical Sciences (Grant Number 93-01-01-8106).
Author information
Authors and Affiliations
Contributions
DM contributed to study design, analysis and interpretation of data. SN and RM contributed to performing the research. KM and GH contributed to interpretation of data and critically revision of the manuscript. AN contributed to study design, analysis and interpretation of data, writing paper and performing the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
This study was performed according to the ethical standards of the Ethical Committee of Shiraz University of Medical Sciences and in compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Immunohistochemical staining of the FOXP3+ Tregs in the lymph node (LN) specimen. Hematoxylin and eosin (H&E)-stained formalin-fixed and paraffin-embedded (FFPE) 3µm tissue blocks of normal tonsile and LN of DLBCL patients at relapsed phase were stained with anti-FOXP3 antibody (236A/E7) (ab20034, 1:100, Abcam); Sample with no recognizable staining was known as negative (−); slight staining was known as weakly positive (+); moderate staining was known as moderately positive (++), and high staining was known as strongly positive (+++). (A) normal tonsile, (B) negative, (C) weakly and (D) moderately positive staining of the FOXP3+ Tregs in three relapsed DLBCL patients. The pictures were captured in 200X and 400X magnification by OLYMPUS microscope. The arrows show the presence of FOXP3+ Tregs (JPEG 2959 kb)
Rights and permissions
About this article
Cite this article
Dehghani, M., Shokrgozar, N., Ramzi, M. et al. The impact of selenium on regulatory T cell frequency and immune checkpoint receptor expression in patients with diffuse large B cell lymphoma (DLBCL). Cancer Immunol Immunother 70, 2961–2969 (2021). https://doi.org/10.1007/s00262-021-02889-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02889-5