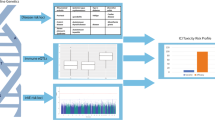
Abstract
Background
Immune checkpoint inhibitors (ICIs) can cause profound immune-related adverse events (irAEs). The host genetic background is likely to play a role in irAE susceptibility because the presentation of toxicity varies among patients and many do not develop irAEs despite continued ICI use. We sought to identify potential genetic markers conferring risk for irAEs.
Methods
We conducted a pilot exploratory study in 89 melanoma patients who received ICIs (44 with irAEs, and 45 without irAEs after at least 1 year from starting treatment). Genotyping was performed using the Infinium Multi-Ethnic Global-8 v1.0 Bead Chip. The genotype data were extracted using PLINK (v1.90b3.34) and processed for quality control. Population structure-based clustering was carried out using IBS matrix, pairwise population concordance test (p < 1 × 10–3), and phenotype distribution for all study participants, resulting in seven population structure-based clusters. In the analytical stage, 599,931 variants in autosomal chromosomes were included for the association study. The association test was performed using an additive genetic model with exact logistic regression, adjusted for age, sex, and population cluster.
Results
A total of 30 variants or single-nucleotide polymorphisms with p < 1 × 10–4 were identified; 12 were associated with an increased risk of irAEs, and the remaining 18 were associated with a decreased risk. Overall, nine of the identified single-nucleotide polymorphisms mapped to eight unique genes that have been associated with autoimmunity or inflammatory diseases.
Conclusion
Several genetic variants associated with irAEs were identified. Additional larger studies are needed to validate these findings and establish their potential functional relevance.
Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T-cell lymphocyte-associated protein-4
- ICI:
-
Immune checkpoint inhibitor
- irAE:
-
Immune-related adverse event
- OR:
-
Odds ratio
- PD-1/PD-L1:
-
Programmed cell death-1/programmed cell death-ligand 1
- SNP:
-
Single-nucleotide polymorphism
References
KEYTRUDA- Pembrolizumab [package insert]. County Cork IM, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s067lbl.pdf. Accessed 1 May 2020
YERVOY-ipilimumab [package insert]. Princeton NBS, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125377s104lbl.pdf. Accessed 1 May 2020
Opdivo-nivolumab [package insert]. Princeton NBMS, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s075lbl.pdf. Accessed 1 May 2020
Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS ONE 11(7):e0160221
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T (2015) Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 13:211
Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X et al (2019) Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 5(7):1008–1019
Le Burel S, Champiat S, Routier E, Aspeslagh S, Albiges L, Szwebel TA et al (2018) Onset of connective tissue disease following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis 77(3):468–470
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S et al (2019) Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Cancer Netw 17(3):255–289
Balint B, Bhatia KP (2016) Stiff person syndrome and other immune-mediated movement disorders—new insights. Curr Opin Neurol 29(4):496–506
Muller R, Heber B, Hashimoto T, Messer G, Mullegger R, Niedermeier A et al (2009) Autoantibodies against desmocollins in European patients with pemphigus. Clin Exp Dermatol 34(8):898–903
Du Y, Wu X, Chen M, Wang W, Xv W, Ye L et al (2017) Elevated semaphorin5A in systemic lupus erythematosus is in association with disease activity and lupus nephritis. Clin Exp Immunol 188(2):234–242
Lyu M, Li Y, Hao Y, Sun T, Liu W, Lyu C et al (2015) Elevated semaphorin 5A correlated with Th1 polarization in patients with chronic immune thrombocytopenia. Thromb Res 136(5):859–864
Gras C, Eiz-Vesper B, Jaimes Y, Immenschuh S, Jacobs R, Witte T et al (2014) Secreted semaphorin 5A activates immune effector cells and is a biomarker for rheumatoid arthritis. Arthritis Rheumatol 66(6):1461–1471
Labiad Y, Venton G, Farnault L, Baier C, Colle J, Mercier C et al (2018) A transcriptomic signature predicting septic outcome in patients undergoing autologous stem cell transplantation. Exp Hematol 65:49–56
Martinez-Hernandez R, Serrano-Somavilla A, Ramos-Levi A, Sampedro-Nunez M, Lens-Pardo A, Munoz De Nova JL et al (2019) Integrated miRNA and mRNA expression profiling identifies novel targets and pathological mechanisms in autoimmune thyroid diseases. EBioMedicine 50:329–342
Glawe JD, Mijalis EM, Davis WC, Barlow SC, Gungor N, McVie R et al (2013) SDF-1-CXCR4 differentially regulates autoimmune diabetogenic T cell adhesion through ROBO1-SLIT2 interactions in mice. Diabetologia 56(10):2222–2230
Aslam MM, John P, Fan KH, Bhatti A, Jahangir S, Feingold E et al (2019) Exploration of shared genetic susceptibility loci between type 1 diabetes and rheumatoid arthritis in the Pakistani population. BMC Res Notes 12(1):544
Inshaw JRJ, Cutler AJ, Crouch DJM, Wicker LS, Todd JA (2020) Genetic variants predisposing most strongly to type 1 diabetes diagnosed under age 7 years lie near candidate genes that function in the immune system and in pancreatic beta-cells. Diabetes Care 43(1):169–177
Bins S, Basak EA, El Bouazzaoui S, Koolen SLW, Oomen-de Hoop E, van der Leest CH et al (2018) Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br J Cancer 118(10):1296–1301
Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA (2019) Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology 58(3):476–480
Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML (2019) Mechanisms of immune-related adverse events associated with immune checkpoint blockade: using germline genetics to develop a personalized approach. Genome Med 11(1):39
Chat V, Ferguson R, Simpson D, Kazlow E, Lax R, Moran U et al (2019) Autoimmune genetic risk variants as germline biomarkers of response to melanoma immune-checkpoint inhibition. Cancer Immunol Immunother 68(6):897–905
Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W et al (2012) PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother 35(2):169–178
von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B et al (2009) CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med 7:35
Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM (2014) Th17 cells in cancer: the ultimate identity crisis. Front Immunol 5:276
Gutierrez-Arcelus M, Rich SS, Raychaudhuri S (2016) Autoimmune diseases—connecting risk alleles with molecular traits of the immune system. Nat Rev Genet 17(3):160–174
Okazaki T, Honjo T (2006) The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 27(4):195–201
Scalapino KJ, Daikh DI (2008) CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev 223:143–155
Schildberg FA, Klein SR, Freeman GJ, Sharpe AH (2016) Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 44(5):955–972
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP et al (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270(5238):985–988
Barreto M, Santos E, Ferreira R, Fesel C, Fontes MF, Pereira C et al (2004) Evidence for CTLA4 as a susceptibility gene for systemic lupus erythematosus. Eur J Hum Genetics 12(8):620–626
Lee YH, Bae SC, Kim JH, Song GG (2015) Meta-analysis of genetic polymorphisms in programmed cell death 1. Associations with rheumatoid arthritis, ankylosing spondylitis, and type 1 diabetes susceptibility. Z Rheumatol 74(3):230–239
Lee YH, Kim JH, Seo YH, Choi SJ, Ji JD, Song GG (2014) CTLA-4 polymorphisms and susceptibility to inflammatory bowel disease: a meta-analysis. Hum Immunol 75(5):414–421
Lee YH, Woo JH, Choi SJ, Ji JD, Song GG (2009) Association of programmed cell death 1 polymorphisms and systemic lupus erythematosus: a meta-analysis. Lupus 18(1):9–15
Li G, Shi F, Liu J, Li Y (2014) The effect of CTLA-4 A49G polymorphism on rheumatoid arthritis risk: a meta-analysis. Diagn Pathol 9:157
Liu JL, Zhang FY, Liang YH, Xiao FL, Zhang SQ, Cheng YL et al (2009) Association between the PD1.3A/G polymorphism of the PDCD1 gene and systemic lupus erythematosus in European populations: a meta-analysis. J Eur Acad Dermatol Venereol 23(4):425–432
Wu J, Zhang L, Zhou Y (2016) The association between CTLA-4 (+49 A/G) polymorphism and susceptibility to ankylosing spondylitis: a meta-analysis. Int J Rheum Dis 19(12):1237–1243
Queirolo P, Dozin B, Morabito A, Banelli B, Carosio R, Fontana V et al (2018) CTLA-4 gene variant − 1661A>G may predict the onset of endocrine adverse events in metastatic melanoma patients treated with ipilimumab. Eur J Cancer 97:59–61
Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C et al (2018) Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 77(3):393–398
Magis Q, Gaudy-Marqueste C, Basire A, Loundou A, Malissen N, Troin L et al (2018) Diabetes and blood glucose disorders under Anti-PD1. J Immunother 41(5):232–240
Brownlie RJ, Zamoyska R, Salmond RJ (2018) Regulation of autoimmune and anti-tumour T-cell responses by PTPN22. Immunology 154(3):377–382
Judd J, Zibelman M, Handorf E, O’Neill J, Ramamurthy C, Bentota S et al (2017) Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 22(10):1232–1237
Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE et al (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13(22 Pt 1):6681–6688
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R et al (2018) Association of immune-related adverse events with Nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378
Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V et al (2018) Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359(6375):582–587
Liu QF, Li Y, Zhao QH, Wang ZY, Hu S, Yang CQ et al (2015) Association of STAT4 rs7574865 polymorphism with susceptibility to inflammatory bowel disease: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 39(5):627–636
Ng SC, Tsoi KK, Kamm MA, Xia B, Wu J, Chan FK et al (2012) Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis 18(6):1164–1176
Siakavellas SI, Bamias G (2018) Checkpoint inhibitor colitis: a new model of inflammatory bowel disease? Curr Opin Gastroenterol 34(6):377–383
Dougan M (2017) Checkpoint blockade toxicity and immune homeostasis in the gastrointestinal tract. Front Immunol 8:1547
Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV et al (2018) Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 3(1):e000278
Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA et al (2018) Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis 24(8):1695–1705
Acknowledgements
We are grateful to Erica Goodoff, from the Department of Scientific Publications, Research Medical Library at The University of Texas MD Anderson Cancer Center for her valuable contribution. We are also grateful to Haidee Chancoco, from the Biospecimen Extraction Resource of MD Anderson Cancer Center for samples processing and DNA extraction.
Funding
This study was funded by a Thrive grant from the Health and Environmental Science Institute to perform this study (Grant Thrive FP00000078). The funding agency had no role in the study’s design; collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this paper for publication. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672 and CA016672 (ATGC).
Author information
Authors and Affiliations
Contributions
MES-A and SSS had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis. Study concept and design: NA, SSS, MES-A. Acquisition of data: NA, AD. Analysis and interpretation of data: NA, RKY. Quality assessment: RKY. Drafting of the manuscript: NA. Critical revision of the manuscript for important intellectual content: AD, RKY, AF, LAC, JHT, RD, VS, SSS, MES-A. Statistical analysis: NA, RKY. Administrative, technical, or material support: SSS, MES-A. Study supervision: SSS, MES-A. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. IRB number: PA16-0928. All patients signed an informed consent prior to study participation.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The is the first pilot genome-wide study to identify genetic variants that may be associated with the risk of developing immune-related adverse events in melanoma patients treated with checkpoint inhibitors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdel-Wahab, N., Diab, A., Yu, R.K. et al. Genetic determinants of immune-related adverse events in patients with melanoma receiving immune checkpoint inhibitors. Cancer Immunol Immunother 70, 1939–1949 (2021). https://doi.org/10.1007/s00262-020-02797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02797-0