Abstract
Immune checkpoint blocking (ICB) is a promising new tool of cancer treatment. Yet, the underlying therapeutic mechanisms are not fully understood. Here we investigated the role of dendritic cells (DCs) for the therapeutic effect of ICB in a λ-MYC-transgenic mouse model of endogenously arising B-cell lymphoma. The growth of these tumors can be effectively delayed by antibodies against CTLA-4 and PD-1. Tumor-infiltrating DCs from mice having received therapy showed an upregulation of costimulatory molecules as well as an augmented IL-12/IL-10 ratio as compared to untreated controls. Both alterations seemed to be induced by interferon-γ (IFN-γ), which is upregulated in T cells and natural killer cells upon ICB. Furthermore, the enhanced IL-12/IL-10 ratio, which favors Th1-prone antitumor T-cell responses, was a consequence of direct interaction of ICB antibodies with DCs. Importantly, the capability of tumor-infiltrating DCs of stimulating peptide-specific or allogeneic T-cell responses in vitro was improved when DCs were derived from ICB-treated mice. The data indicate that ICB therapy is not only effective by directly activating T cells, but also by triggering a complex network, in which DCs play a pivotal role at the interface between innate and adaptive antitumor responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past years, immune checkpoint blockade (ICB) has substantially advanced the field of cancer immunotherapy in the clinics. Approaches have been conceived to block counter-regulatory molecules like cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) or programmed death-1 (PD-1), which are often associated with tumor-infiltrating T cells (TILs) whose functions are silenced or exhausted in the tumor microenvironment [1,2,3,4,5,6]. Although monoclonal antibodies (mAbs) targeting CTLA-4 or PD-1, such as Ipilimumab or Nivolumab and Pembrolizumab, have already been successfully established as new tools for treating several types of cancer including lymphoma (reviews in [7,8,9,10]), the underlying tumor-suppressive mechanisms are not yet completely understood.
While the rationale of ICB originally aimed at re-activating TILs against malignant cells by directly disrupting the interaction between the counter-regulatory receptors on T cells and their ligands, other pathways and cell populations are also involved in mediating the therapeutic effect. Thus, in a λ-MYC transgenic mouse model of endogenously arising B-cell lymphoma [11], ICB-induced long-term tumor suppression was not only due to the activation of T cells, but also to natural killer (NK) cells [12]. Interferon-γ (IFN-γ) and tumor necrosis factor (TNF) were strictly required for tumor control in this model [13]. These cytokines are capable of activating the p16Ink4a-Rb and the p53-p21 signaling pathways [13, 14], thereby causing cell cycle arrest of cancer cells [14, 15]. Hence, the effect of ICB was additionally mediated by cytokine-induced senescence of malignant cells [12, 13]. IFN-γ, which was required for this pathway, was not only provided by T cells upon ICB, but also by NK cells, which turned out to be critical for the therapeutic efficacy in the lymphoma model. The NK cells were directly stimulated by the anti-CTLA-4/anti-PD-1 mAbs to produce IFN-γ, thereby contributing to senescence induction in tumor cells [12].
Generally, NK-cell activation can initiate a cascade that bridges the innate and the adaptive immune response. Activated NK cells can lyse tumor cells [16,17,18], which leads to engulfment of antigens by dendritic cells (DCs) and presentation of immunogenic peptides to T cells in a major histocompatibility complex- (MHC-) restricted manner. In addition, the cytokine IFN-γ, which is upregulated in activated NK cells, stimulates DCs to express IL-12, which eventually fosters a long-lasting T-cell-dependent antitumor memory response [19, 20]. Although IFN-γ expression by NK cells is severely impaired in untreated endogenous lymphoma [21], the NK/DC/T-cell axis can be triggered even in this setting [16] and might also become effective after ICB-induced IFN-γ upregulation.
The expression pattern of the instructive cytokines IL-12 and IL-10 in DCs is decisive for directing immune responses towards a Th1 or Th2 type. Tumor-infiltrating DCs (TIDCs) frequently undergo a shift from IL-12 to IL-10 expression [22,23,24] (review in [25]). This bias may contribute to immune escape of tumors because T-cell-mediated tumor rejection requires a Th1-driven response involving IFN-γ and IL-12 rather than a Th2-prone milieu, which is promoted by IL-10 [14, 15, 26, 27].
Having previously shown that multiple mechanisms, which also involve NK cells, are elicited by ICB therapy, we now asked the question whether DCs contribute to the therapeutic potential of anti-CTLA-4 and anti-PD-1 mAbs in the λ-MYC lymphoma model. We show that DC functions are favorably affected by ICB, an effect that is at least partially dependent on IFN-γ and may endorse adaptive antitumor immune responses.
Materials and methods
Animal experiments
C57BL/6 wildtype (WT) as well as λ-MYC mice were bred in our own animal facility. BALB/c mice were purchased from Charles River Laboratories, and OT-II mice were kindly provided by Prof. Dr. Brocker, Institute of Immunology, München. Breeding of mice and all experiments were only done after approval by the responsible authority.
For ICB therapy, λ-MYC Mice were injected i.p. with 100 µg each of anti-PD-1 (J43; BioXCell, West Lebanon, U.S.A.) and anti-CTLA-4 mAbs (UC10-4B9; BioLegend, San Diego, U.S.A.) starting at an age of 60 days. Treatment was repeated three times every 10 days. To neutralize IFN-γ during ICB therapy, mice received 500 µg, 300 µg, 150 µg and again 150 µg of the anti-IFN-γ mAb XMG-1.2 (Core facility mAb, Helmholtz-Zentrum München) i.p. 6–12 h before each ICB mAb injection.
Depletion of NK cells was performed by i.p. application of 50 μg polyclonal anti-Asialo-GM1 (Thermo Fisher Scientific, Waltham, U.S.A.) in weekly to biweekly intervals. Effective NK-cell depletion was verified by examining splenocytes for NK1.1 expression.
Fluorescence-activated cell sorting (FACS)
After homogenizing spleens, cells were treated with erythrocyte lysis buffer, meshed through a 35-μm cell strainer, washed with PBS, and analyzed by FACS.
DCs were phenotyped by using fluorochrome-labeled mAbs directed against CD11c (N418; BioLegend), CD80 (16-10A1; BD Pharmingen, Heidelberg, Germany), CD86 (PO3; Thermo Fisher Scientific), PD-1 (J43, Thermo Fisher Scientific), CTLA-4 (UC10-4B9; BioLegend), CD83 (Michel-19; BioLegend), CD40 (3/23; BioLegend), MHCII (2G9; BD Pharmingen) and PD-L1 (MIH6; AbD Serotec, Puchheim, Germany). Dead cells were excluded using the LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit (Thermo Fisher Scientific). Following mAb labeling for 30 min at 4 °C and final washing, cells were analyzed on an LSR II flow cytometer.
To measure intracellular cytokines, cells were stimulated with PMA and ionomycin (1 μg/ml each; Sigma-Aldrich, Taufkirchen, Germany) for 4 h in the presence of 3 μg/ml Brefeldin A (Thermo Fisher Scientific), labeled for CD11c, subjected to fixation and permeabilization and subsequently stained with mAbs against IL-10 (JES5-16E3; BD Pharmingen) and IL-12 (C15.6; BD Pharmingen). IFN-γ in T cells was measured by using fluorochrome-labeled anti-IFN-γ (XMG-1.2, BioLegend) and counterstaining with anti-CD4 (RM4-5, eBioscience, Frankfurt, Germany).
Functional assays in vitro
Immune cells were isolated from tumor-infiltrated as well as WT spleens. DCs and T cells were purified by immunomagnetic separation using anti-CD11c microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and the CD4+ T Cell Isolation Kit (Stemcell Technologies, Vancouver, Canada), respectively. Purity was > 98%.
5 × 105 DCs derived from λ-MYC spleens were incubated in 200 μl RPMI 1640 medium supplemented with 10% FCS in the presence of 0.1 µg/ml each of anti-PD-1 and anti-CTLA-4 mAbs for 72 h in 96-well plates. Then, IL-12 and IL-10 expression was determined by FACS analyses as described above.
For peptide-specific stimulation, 5 × 104 DCs from untreated or ICB-treated λ-MYC or from WT mice were incubated with MHC II-restricted OVA323-339 peptides (GenWay Biotech, San Diego, U.S.A.) at a concentration of 1 μg/ml for 3 h. Then, peptide-pulsed DCs were coincubated with 105 CD4+ T cells derived from OT-II mice. For allostimulation, 104 DCs freshly isolated from untreated or ICB-treated λ-MYC or from WT mice were cocultured with 2 × 105 T cells from BALB/c mice.
All T-cell stimulation experiments were done in 200 μl RPMI 1640 medium supplemented with 10% FCS in 96-well plates. Supernatants were taken after 3 and 5 days and analyzed for IFN-γ concentrations by enzyme-linked immunosorbent assay using the Mouse IFN-γ ELISA Ready-SET-Go kit (eBioscience).
Statistics
Differences between two independent groups were assessed using the unpaired Student’s t test or Mann–Whitney test. All results were expressed as means ± SEM. Data analysis was performed with Prism 5.0 software (GraphPad).
Results
Increased expression of costimulatory molecules on TIDCs after ICB
λ-MYC mice, which are of C57BL/6 origin, constitutively express a transgenic c-MYC oncogene in a B-cell-specific manner, which leads to the development of endogenous B-cell lymphomas in 100% of mice [11]. Tumors grow in spleen and lymph nodes and lead to death between about day 70 and day 140 after birth. As shown earlier, DCs in the tumor microenvironment show phenotypic and functional alterations such as a reduced IL-12/IL-10 ratio and an impaired capability of stimulating T cells, although the expression of costimulatory molecules was increased [24]. Furthermore, the fraction of CD11clow DCs, which are considered as a T-cell-inhibiting DC subset [28], was increased in comparison to the CD11chigh population.
We furthermore showed that combined treatment with anti-PD-1 and anti-CTLA-4 mAbs significantly delays tumor development and even gives rise to long-time survivors, while therapy with single mAbs remains uneffective [13]. The frequencies of T lymphocytes, which are greatly diminished in the tumors, partly recover by ICB therapy [12]. While in diseased animals, the organ architecture is destroyed, lymphoid organs from ICB-treated mice, which are still healthy, show a normal distribution of T and B cells [13].
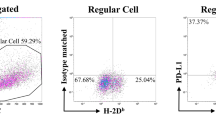
The therapeutic effect was associated with increased IFN-γ release by T cells as well as by NK cells [12]. To elucidate the impact of ICB on DCs, we phenotypically characterized spleen-derived TIDCs from mice that received ICB therapy and therefore showed delayed tumor growth. The comparison with untreated animals, whose tumor burdens were identical but only appeared at earlier time points than in the treated group, revealed that ICB significantly reduced the ratio of CD11clow to CD11chigh cells (Fig. 1a, b). As the costimulatory molecules CD80 and CD86 expressed on DCs, which are most important for activating specific T cells and preventing T-cell anergization, can be upregulated by IFN-γ [24], we particularly analyzed these molecules on TIDCs. It turned out that ICB further increased the expression of CD80 and CD86, at least in the CD11clow subset (Fig. 1c, d). With regard to other maturation-associated markers, ICB showed variable effects. While slight increases were detected for CD40 and MHC class II, CD83 was markedly reduced after ICB, a finding that cannot be explained for the time being (Supplementary Fig. 1). Of note, PD-L1, which interacts with PD-1 and thereby impairs T-cell functions, was significantly reduced on CD11clow TIDCs from ICB-treated mice.
Characterization of surface markers expressed on spleen DCs from tumor-bearing λ-MYC mice that did or did not receive ICB treatment. a Distribution of CD11clow and CD11chigh DCs. In the example shown, the ratio CD11clow/CD11chigh is 5.12 (untreated) and 2.87 (treated). b Compilation of CD11clow/CD11chigh ratios from untreated (n = 24) and treated mice (n = 7). WT mice are included for comparison. The difference between the treated and the untreated group is significant with P < 0.05. c, d Percentages of CD80+ and CD86+ cells in the CD11clow and CD11chigh fractions. 4 and 9 animals were analyzed in the ICB and in the control group, respectively. In the CD11clow population, the differences are significant with P < 0.05
ICB affects the pattern of instructive cytokines expressed by TIDCs via IFN-γ
The expression profile of instructive cytokines in TIDCs from untreated tumor mice displayed a reduced IL-12/IL-10 quotient, which predicts a compromised Th1/Tc1 polarization of T-cell responses [24]. To answer the question whether ICB affects this imbalance of DC-derived instructive cytokines, we analyzed intracellular cytokine levels in TIDCs from spleens of ICB-treated λ-MYC animals. After ICB, individual mice showed variable results with increased IL-12 and reduced IL-10 expression (Fig. 2a, b), which resulted in an increased IL-12/IL-10 ratio, even though this was not statistically significant (Fig. 2c).
Impact of ICB therapy on expression patterns of instructive cytokines in TIDCs. a, b Typical result of intracellular staining of IL-12 and IL-10 in CD11c+ spleen cells. Similar patterns were detected in CD11clow and CD11chigh cells. c Compilation of IL-12/IL-10 ratios in TIDCs from animals that received ICB therapy or were left untreated. In some animals, ICB was combined with neutralization of IFN-γ using the mAb XMG-1.2. Up to 14 mice were used per group. d Percentages of IFN-γ+ cells in the CD4+ T-cell population from spleens of the indicated mouse groups. 4–5 mice were included in each group. The difference between the ICB-treated group and all other settings is significant with P < 0.01. In the CD8+ subset, the effect of ICB in terms of IFN-γ induction was far less pronounced [12]
The decreased IL-12/IL-10 ratio in DCs from untreated tumor-bearing mice is likely a consequence of IFN-γ suppression, which occurs in the microenvironment of growing λ-MYC lymphomas [21]. Since enhanced amounts of IFN-γ were provided by NK [12] and T cells (Fig. 2d) after delivery of anti-PD-1 and anti-CTLA-4 mAbs, we speculated that the higher IL-12/IL-10 ratio following ICB therapy is induced by this cytokine. Therefore, we analyzed λ-MYC mice that were treated with ICB under mAb-dependent neutralization of IFN-γ. After blocking of IFN-γ, the ICB-induced increase of the IL-12/IL-10 ratio in TIDCs was indeed attenuated (Fig. 2c). This suggests that IFN-γ plays a role for transmitting ICB-dependent effects to DCs. Depleting IFN-γ during ICB therapy also resulted in an impaired T-cell-intrinsic capability of producing IFN-γ (Fig. 2d). As the same result was obtained by depleting NK cells (Fig. 2d), the latter cell population seems to be necessary for providing IFN-γ, which, in turn, supports the T-cell response. IFN-γ may exert this effect directly, but presumably also through alteration of instructive cytokines derived from DCs.
Direct interaction of ICB mAbs with DCs
These results did not preclude that direct binding of ICB mAbs to the DC surface may also occur. Therefore, we tested first the expression of immune checkpoint molecules by TIDCs. In comparison to WT DCs, TIDCs from λ-MYC animals exhibited strong expression of PD-1 and CTLA-4 (Fig. 3a, b). This finding prompted us to determine the cytokine profile of purified CD11c+ DCs after incubation with anti-PD1 and anti-CTLA-4 mAbs in vitro. After mAb treatment, the IL-12/IL-10 quotient was indeed enhanced (Fig. 3c). As enrichment of DCs via CD11c also yields low numbers of T and NK cells expressing CD11c (not shown), IFN-γ secreted by the latter cells may be responsible for this effect, but it cannot be ruled out that ICB mAbs may have impact on TIDC-derived instructive cytokines by direct interaction with DC surface molecules.
Direct interaction of anti-PD-1 and anti-CTLA-4 mAbs with DCs. a, b Exemplary results showing higher levels of PD-1 and CTLA-4 expressed on λ-MYC TIDCs than on DCs from WT mice. c IL-12/IL-10 ratios in purified CD11c+ cells with or without incubation with ICB mAbs for 72 h in vitro (n = 5). Identical results were obtained in CD11clow and CD11chigh cells
Functional restoration of TIDCs by ICB therapy
Since the T-cell-stimulatory capacity of λ-MYC tumor-derived TIDCs was compromised [24], we asked the question whether the tumor-dependent functional impairment of DCs can be attenuated by ICB. Therefore, we examined the function of DCs from ICB-treated mice in terms of stimulating naïve T cells in vitro. For testing peptide-specific reactivity, we incubated syngeneic OT-II CD4+ T cells, which express OVA-specific T-cell receptors, with TIDCs that were pulsed with appropriate MHC class II-restricted OVA peptides. Whereas IFN-γ secretion by T cells was impaired when stimulated with TIDCs from untreated λ-MYC mice, IFN-γ production by CD4+ T cells was restored when DCs were derived from donors that had been subjected to ICB therapy before (Fig. 4a). Similar results were obtained when normal CD4+ T cells were stimulated in an allogeneic setting using BALB/c mice as donors of responder cells (Fig. 4b).
In-vitro stimulation of CD4+ T cells by DCs from WT mice or from tumor-bearing MYC animals without or after ICB therapy. a OT-II-specific T cells were stimulated with OVA peptide-loaded DCs (n = 3). b CD4+ T-cell response after allogeneic stimulation (n = 6). Supernatants were analyzed for IFN-γ concentrations after 5 days. The WT and the MYC groups without therapy are different from the ICB groups with P < 0.05
Discussion
The mechanisms underlying ICB-mediated tumor suppression are complex (Fig. 5). In the λ-MYC lymphoma model, we showed that IFN-γ is strictly required for successful therapy [12, 13]. This cytokine not only fosters tumor-protective Th1/Tc1 responses but also triggers several non-immunologic pathways leading to tumor growth inhibition [13, 14, 29] including intrinsic senescence induction in tumor cells [12,13,14]. Furthermore, NK cells activated by ICB were necessary for prolonging the survival of λ-MYC mice [12]. NK cells may be a critical source of IFN-γ that was needed for the therapeutic effect. The data presented here suggest that DCs are also involved in ICB therapy and that activation of an NK cell/DC axis may additionally endorse tumor-directed T-cell responses (Fig. 5).
The maturation status of TIDCs, hence the expression of costimulatory molecules like CD80 and CD86, seems to be determined by the tumor entity. Thus, in some endogenous mouse tumor models, DCs displayed a downregulation of costimulatory molecules [23, 30], whereas a mature phenotype was observed in other neoplasias including the λ-MYC lymphoma [24, 31]. It has been shown that soluble IFN-γ or IFN-γ-producing NK cells are capable of increasing the CD80 and CD86 expression on DCs [24]. Therefore, it was not surprising that ICB-dependent IFN-γ induction in T cells and in NK cells resulted in an even higher density of CD80 and CD86 on TIDCs (Fig. 1c, d, Fig. 5). However, it is an open question whether this additional upregulation is relevant for the success of ICB therapy, because, in untreated tumor-bearing λ-MYC mice, costimulatory molecules on DCs were also elevated in comparison to normal mice and an anergization of T cells owing to a lack of costimuli is, therefore, unlikely to account for immune escape [24].
Instead, a beneficial effect might be mediated by an ICB-induced increase of the IL-12/IL-10 ratio in λ-MYC TIDCs (Fig. 2c). In untreated tumor-bearing animals, this parameter was significantly reduced compared with healthy WT mice. This could be assigned to the action of IL-10, which was primarily produced by the tumor cells [24]. The capability of ICB therapy of reversing the cytokine imbalance in TIDCs may be due to direct mAb binding to PD-1 and CTLA-4 expressed on the DC surface (Fig. 3) as well as to an indirect effect mediated by IFN-γ [19, 20]. In a model of ovarian cancer, TIDCs also expressed PD-1 molecules on their surface, which were recognized by anti-PD-1 mAbs [32].
Experiments in vitro could not exclude that direct interactions of ICB mAbs with TIDCs expressing CTLA-4 and PD-1 may have impact on the IL12/IL-10 ratio (Fig. 3). In the in vivo situation, however, the cytokine expression pattern seemed to be mainly determined by IFN-γ, because ablation of IFN-γ during ICB therapy blocked the ICB-induced increase of the IL-12/IL-10 ratio (Fig. 2c). IFN-γ but not direct mAb binding to DCs may therefore be necessary in the in vivo setting, which is in accordance with another investigation, where DCs were similarly stimulated to express IL-12 via IFN-γ [33]. In the latter study, IFN-γ was derived from anti-PD-1-activated CD8+ T cells and eventually gave rise to activation of effector T cells. Further studies conducted in mouse tumor models indicate an activation of CD4+ T cells, which again stimulated DCs to express IL-12 [34]. The improved T cell-stimulatory capacity of λ-MYC TIDCs detected in vitro (Fig. 4), which was also seen in an HPV16 tumor model after anti-PD1/anti-CTLA-4 treatment [35], may be due to the altered cytokine expression pattern, but other mechanisms cannot be precluded.
Recent studies demonstrated an antimetastatic effect of NK cells induced by ICB in combination with IL-12 treatment [36] and ameliorated NK-cell functions as a result of reactivating specific CD4+ T cells [37]. Hence, reinvigoration of an exhausted adaptive immune response can give rise to an improved innate response [37]. Our results suggest that an additional, inverse pathway may exist, which is initiated by IFN-γ produced by activated NK cells, for example (Fig. 5). The lymphoma model presented here may reflect the situation in human melanoma where NK cells controlled DCs and stimulated a T-cell response through an NK cell/DC axis [38]. In summary, ICB therapy not only reactivates exhausted T cells but may also promote additional pathways, in which IFN-γ and DCs play a significant role.
References
Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA (1994) CTLA-4 can function as a negative regulator of T cell activation. Immunity 1:405–413
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ (2002) The interaction properties of costimulatory molecules revisited. Immunity 17:201–210
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12:492–499
Grosso JF, Jure-Kunkel MN (2013) CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 13:5
Callahan MK, Postow MA, Wolchok JD (2016) Targeting T cell co-receptors for cancer therapy. Immunity 44:1069–1078
Dyck L, Mills KHG (2017) Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 47:765–779
LaFleur MW, Muroyama Y, Drake CG, Sharpe AH (2018) Inhibitors of the PD-1 pathway in tumor therapy. J Immunol 200:375–383
Xu-Monette Z, Young KH (2018) PD-1 expression and clinical PD-1 blockade in B-cell lymphoma. Blood 131:68–83
Kovalchuk AL, Qi CF, Torrey TA, Taddesse-Heath L, Feigenbaum L, Park SS, Gerbitz A, Klobeck G, Hörtnagel K, Polack A, Bornkamm GW, Janz S, Morse HC 3rd (2000) Burkitt lymphoma in the mouse. J Exp Med 192:1183–1190
Ahmetlić F, Fauser J, Riedel T, Flessner C, Hömberg N, Hennel R, Brenner E, Braumüller H, Lauber K, Röcken M, Mocikat, R. Therapy of B-cell lymphoma by immune checkpoint inhibitors: The role of T cells, NK cells and cytokine-induced tumor senescence. manuscript in revision
Brenner E, Schörg BF, Ahmetlić F, Wieder T, Hilke FJ, Simon N, Schroeder C, Demidov G, Riedel T, Fehrenbacher B, Schaller M, Forschner A, Eigentler T, Niessner H, Sinnberg T, Böhm KS, Hömberg N, Braumüller H, Dauch D, Zwirner S, Zender L, Sonanini D, Geishauser A, Bauer J, Eichner M, Jarick K, Beilhack A, Biskup S, Döcker D, Schadendorf D, Quintanilla-Martínez L, Pichler B, Kneilling M, Mocikat R, Röcken M (2020) Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat Commun 11:1335
Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, Mocikat R, Braungart K, Mehra T, Fehrenbacher B, Berdel J, Niessner H, Meier F, van den Broek M, Häring HU, Handgretinger R, Quintanilla-Martinez L, Fend F, Pesic M, Bauer J, Zender L, Schaller M, Schulze-Osthoff K, Röcken M (2013) T-helper-1-cell cytokines drive cancer into senescence. Nature 494:361–365
Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Förster I, Huss R, Weber WA, Kneilling M, Röcken M (2008) TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13:507–518
Hömberg N, Adam C, Riedel T, Brenner C, Flatley A, Röcken M, Mocikat R (2014) CD40-independent natural killer-cell help promotes dendritic cell vaccine-induced T-cell immunity against endogenous B-cell lymphoma. Int J Cancer 135:2825–2833
Belting L, Hömberg N, Przewoznik M, Brenner C, Riedel T, Flatley A, Polić B, Busch DH, Röcken M, Mocikat R (2015) Critical role of the NKG2D receptor for NK cell-mediated control and immune escape of B-cell lymphoma. Eur J Immunol 45:2593–2601
Cerwenka A, Baron JL, Lanier LL (2001) Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A 98:11521–11526
Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, Bubeck A, Louis J, Mailhammer R, Riethmüller G, Koszinowski U, Röcken M (2003) Natural killer cells activated by MHC class I-low targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561–569
Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, Kriegeskorte A, Busch DH, Röcken M, Mocikat R (2005) DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106:338–344
Przewoznik M, Hömberg N, Naujoks M, Pötzl J, Münchmeier N, Brenner C, Anz D, Bourquin C, Nelson P, Röcken M, Mocikat R (2012) Recruitment of natural killer cells in advanced stages of endogenously arising B-cell lymphoma: implications for therapeutic cell transfer. J Immunother 35:217–222
Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML (2003) Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 89:1463–1472
Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V (2009) Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res 15:4382–4390
Naujoks M, Weiß J, Riedel T, Hömberg N, Przewoznik M, Nößner E, Röcken M, Mocikat R (2014) Alterations of costimulatory molecules and instructive cytokines expressed by dendritic cells in the microenvironment of an endogenous mouse lymphoma. Cancer Immunol Immunother 63:491–499
Gabrilovich D (2004) Mechanisms and functional significance of tumor-induced dendritic-cell defects. Nat Rev Immunol 4:941–952
Egeter O, Mocikat R, Ghoreschi K, Dieckmann A, Röcken M (2000) Eradication of disseminated lymphomas with CpG-DNA-activated Th1 cells from non-transgenic mice. Cancer Res 60:1515–1520
Ziegler A, Heidenreich R, Braumüller H, Wolburg H, Weidemann S, Mocikat R, Röcken M (2009) EpCAM, a human tumor-associated antigen, promotes Th2 development and tumor immune evasion. Blood 113:3494–3502
Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X (2009) Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol 182:6207–6216
Qin Z, Blankenstein T (2000) CD4+ T cell-mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity 12:677–686
Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, ColomboO´Garra MPA, Trinchieri G, Caux C (2002) Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med 196:541–549
Norian LA, Rodriguez PC, O´Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM (2009) Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res 69:3086–3094
Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, Kalli KR, Goode EL, Block MS, Cannon MJ, Knutson KL (2017) IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res 77:6667–6678
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, Freeman GJ, Warren SE, Ong S, Browning E, Twitty CG, Pierce RH, Le MH, Algazi AP, Daud AI, Pai SI, Zippelius A, Weissleder R, Pittet MJ (2018) Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 49:1148–1161
Beavis PA, Henderson MA, Giuffrida L, Davenport AJ, Petley EV, House IG, Lai J, Sek K, Milenkovski N, John LB, Mardiana S, Slaney CY, Trapani JA, Loi S, Kershaw MH, Hayners NM, Darcy PK (2018) Dual PD-1 and CTLA-4 checkpoint blockade promotes antitumor responses through CD4+Foxp3- cell-mediated modulation of CD103+ dendritic cells. Cancer Immunol Res 6:1069–1081
Sun NY, Chen YL, Lin HW, Chiang YC, Chang CF, Tai YJ, Chen CA, Sun WZ, Chien CL, Cheng WF (2019) Immune checkpoint Ab enhances the antigen-specific antitumor effects by modulating both dendritic cells and regulatory T lymphocytes. Cancer Lett 444:20–34
Ohs I, Ducimetière L, Marinho J, Kulig P, Becher B, Tugues S (2017) Restoration of natural killer cell antimetastatic activity by IL12 and checkpoint blockade. Cancer Res 77:7059–7071
Porichis F, Hart MG, Massa A, Everett HL, Morou A, Richard J, Brassard N, Veillette M, Hassan M, Le Ly N, Routy J-P, Freeman GJ, Dubé M, Finzi A, Kaufmann DE (2018) Immune checkpoint blockade restores HIV-specific CD4 T cell help for NK cells. J Immunol 201:971–981
Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, Alvarado MD, Wolf DM, Bogunovic D, Bhardwaj N, Daud AI, Ha PK, Ryan WR, Pollack JL, Samad B, Asthana S, Chan V, Krummel MF (2018) A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 24:1178–1191
Acknowledgements
We thank Michael Hagemann and Franziska Liebel for taking care of mice and performing animal experiments and Albert Geishauser for genotyping mice. We are grateful to Nadine Hömberg for formatting work. The study includes parts of the doctoral theses of A.S., F.A., V.B. and T.R. at the Ludwig-Maximilians-Universität München. The work was supported by grants from Deutsche Krebshilfe (70112332, 70112337, 110662 and 110664), Wilhelm-Sander-Stiftung (2012.056.3) and Deutsche Forschungsgemeinschaft (SFB-TR 156 and DFG Ro 764/15-2).
Funding
Open Access funding enabled and organized by Projekt DEAL. The work was supported by grants from Deutsche Krebshilfe (70112332, 70112337, 110662, and 110664), Wilhelm-Sander-Stiftung (2012.056.3) and Deutsche Forschungsgemeinschaft (SFB-TR 156 and DFG Ro 764/15-2).
Author information
Authors and Affiliations
Contributions
A.S., F.A., V.B. and T.R. performed experiments and analyzed data, A.S. contributed to writing the manuscript and drafted the figures, M.R. and R.M. conceived and supervised the study, R.M. wrote the manuscript. All authors agreed to the content and the submission of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Animal experiments were approved by the responsible authority.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scheuerpflug, A., Ahmetlić, F., Bauer, V. et al. The role of dendritic cells for therapy of B-cell lymphoma with immune checkpoint inhibitors. Cancer Immunol Immunother 70, 1343–1350 (2021). https://doi.org/10.1007/s00262-020-02767-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02767-6