Abstract
Pancreatic ductal adenocarcinoma (PDAC) has a heterogeneous tumor microenvironment (TME) comprised of myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages, neutrophils, regulatory T cells, and myofibroblasts. The precise mechanisms that regulate the composition of the TME and how they contribute to radiotherapy (RT) response remain poorly understood. In this study, we analyze changes in immune cell populations and circulating chemokines in patient samples and animal models of pancreatic cancer to characterize the immune response to radiotherapy. Further, we identify STAT3 as a key mediator of immunosuppression post-RT. We found granulocytic MDSCs (G-MDSCs) and neutrophils to be increased in response to RT in murine and human PDAC samples. We also found that RT-induced STAT3 phosphorylation correlated with increased MDSC infiltration and proliferation. Targeting STAT3 using an anti-sense oligonucleotide in combination with RT circumvented RT-induced MDSC infiltration, enhanced the proportion of effector T cells, and improved response to RT. In addition, STAT3 inhibition contributed to the remodeling of the PDAC extracellular matrix when combined with RT, resulting in decreased collagen deposition and fibrotic tissue formation. Collectively, our data provide evidence that targeting STAT3 in combination with RT can mitigate the pro-tumorigenic effects of RT and improve tumor response.





Similar content being viewed by others
References
Garrido-Laguna I, Hidalgo M (2015) Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 12(6):319–334
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA A Cancer J Clin 69(1):7–34
Bailey P et al (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531(7592):47–52
Li X et al (2015) Emerging immune checkpoints for cancer therapy. Acta Oncol 54(10):1706–1713
Huguet F et al (2007) Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 25(3):326–331
Balogh A et al (2013) The effect of ionizing radiation on the homeostasis and functional integrity of murine splenic regulatory T cells. Inflamm Res 62(2):201–212
Cao M et al (2009) Gamma irradiation alters the phenotype and function of CD4+CD25+ regulatory T cells. Cell Biol Int 33(5):565–571
Seifert L et al (2016) Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology 150(7):1659–1672 (e5)
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174
Barcellos-Hoff MH (2010) Stromal mediation of radiation carcinogenesis. J Mammary Gland Biol Neoplasia 15(4):381–387
Barker HE et al (2015) The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15(7):409–425
Ohuchida K et al (2004) Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res 64(9):3215–3222
Neesse A et al (2011) Stromal biology and therapy in pancreatic cancer. Gut 60(6):861–868
Mariathasan S et al (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554(7693):544–548
Petit V et al (2013) Optimization of tumor xenograft dissociation for the profiling of cell surface markers and nutrient transporters. Lab Investig 93(5):611–621
Hadi AM et al (2011) Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 34(4):343–354
Chong J et al (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46(W1):W486–W494
Hillmer EJ et al (2016) STAT3 signaling in immunity. Cytokine Growth Factor Rev 31:1–15
Cursiefen C et al (2004) VEGF—a stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Investig 113(7):1040–1050
Garnett CT et al (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64(21):7985–7994
Lennon S et al (2019) Pancreatic tumor microenvironment modulation by EphB4-ephrinB2 inhibition and radiation combination. Clin Cancer Res 25(11):3352–3365
Chakraborty D et al (2017) Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun 8(1):1130
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 12(6):325–338
Lei J et al (2013) Sdc1 overexpression inhibits the p38 MAPK pathway and lessens fibrotic ventricular remodeling in MI rats. Inflammation 36(3):603–615
Rockey DC, Weymouth N, Shi Z (2013) Smooth muscle alpha actin (Acta 2) and myofibroblast function during hepatic wound healing. PLoS One 8(10):e77166
Hu CE et al (2011) Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol 46(2):156–164
Lesokhin AM et al (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72(4):876–886
Marvel D, Gabrilovich DI (2015) Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig 125(9):3356–3364
Marigo I et al (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 222:162–179
Li H et al (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 182(1):240–249
Murdoch C et al (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8(8):618–631
Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A et al (2010) Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 40(1):22–35
Condamine T et al (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97–110
Movahedi K et al (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111(8):4233–4244
Mao Y et al (2013) Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73(13):3877–3887
Srivastava MK et al (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 70(1):68–77
Hanson EM et al (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 183(2):937–944
Lindau D et al (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138(2):105–115
Gough MJ, Young K, Crittenden M (2013) The impact of the myeloid response to radiation therapy. Clin Dev Immunol 2013:281958
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809
Oweida AHM, Phan A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Vancourt B, Uyanga N, Darragh L, Kim HM, Raben D, Tan AC, Heasley L, Clambey E, Nemenoff R, Karam SD (2018) Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res 24:5368–5380
Hossain DM et al (2013) FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 39(6):1057–1069
Liang H et al (2017) Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun 8(1):1736
Yako YY et al (2016) Cytokines as biomarkers of pancreatic ductal adenocarcinoma: a systematic review. PLoS One 11(5):e0154016
Bauer J et al (2012) Effects of activin and TGFbeta on p21 in colon cancer. PLoS One 7(6):e39381
Miyazono K (2000) Positive and negative regulation of TGF-beta signaling. J Cell Sci 113(Pt 7):1101–1109
Miyazono K, ten Dijke P, Heldin CH (2000) TGF-beta signaling by Smad proteins. Adv Immunol 75:115–157
Apte MV et al (1999) Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44(4):534–541
Shek FW et al (2002) Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160(5):1787–1798
Ijichi H et al (2006) Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 20(22):3147–3160
Kojima K et al (2007) Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res 67(17):8121–8130
Bendell JC et al (2014) Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol 74(1):125–130
Hong D et al (2015) AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med 7(314):314ra185
Acknowledgements
We would like to acknowledge and thank Ionis Pharmaceuticals for providing the mouse surrogate STAT3 ASO. This work was funded in part by a Grant from AstraZeneca. This work was also supported by Cancer Center Support Grant (P30CA046934), R01-DE028282 (Karam), R01-DE028529 (Karam), Paul Sandoval Funds (Mueller), RSNA Resident Research Grant (Mueller), Cancer League of Colorado Grant (Mueller) and by the Wings of Hope Foundation (Karam, Goodman).
Funding
This work was supported by Cancer Center Support Grant (P30CA046934), R01-DE028282 (Karam), R01-DE028529 (Karam), Paul Sandoval Funds (Mueller), RSNA Resident Research Grant (Mueller), Cancer League of Colorado Grant (Mueller) and by the Wings of Hope Foundation (Karam, Goodman).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All protocols for animal tumor models were approved by the IACUC of the University of Colorado Denver. Mice exhibiting signs of morbidity according to the guidelines set by the Institutional Animal Care and Use Committee (IACUC) were sacrificed immediately.
Informed consent
All human studies were performed after approval by the University of Colorado institutional review board (COMIRB16-1139) and written informed consent was obtained from all patients as dictated by the study protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
262_2020_2701_MOESM1_ESM.pdf
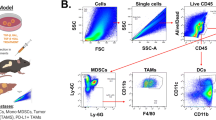
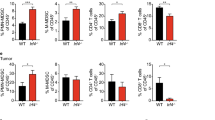
Supplementary Figure 1 – (a) Identification and gating of immune populations on flow cytometry. For MDSC gating, the following criteria were used as previously described [33, 53]. In mouse samples, total MDSCs were defined as CD11b+Gr1+ cells. The two major subsets of MDSCs were differentiated by variable expression of the Gr1 marker (comprised of Ly6C and Ly6G). M-MDSCs were defined as CD11b+Ly6ChiLy6G– and G-MDSCs as CD11b+Ly6CloLy6G+. In human samples, only total MDSCs were analyzed and defined as CD11b+CD33+. (b) Effect of RT on the proportion of intratumoral T cells populations. (c) Analysis of pSTAT3 expression in various cell populations from FC1242 tumors. (d) Effect of RT on pSTAT3 expression in T cell populations. (PDF 295 kb)
262_2020_2701_MOESM2_ESM.pdf
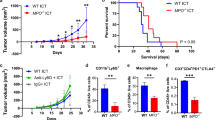
Supplementary Figure 2 – Analysis of tumor growth in (a) FC1242 and (b) PK5L1940 tumor-bearing mice. (c) Analysis of tumor growth using STAT3 ASO alone. (PDF 227 kb)
262_2020_2701_MOESM3_ESM.pdf
Supplementary Figure 3 – (a) Relative M1/M2 ratios following STAT3 ASO + RT treatment. (b) T cell pSTAT3 expression levels following STAT3 ASO + RT treatment. (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Oweida, A.J., Mueller, A.C., Piper, M. et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol Immunother 70, 989–1000 (2021). https://doi.org/10.1007/s00262-020-02701-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02701-w