Abstract
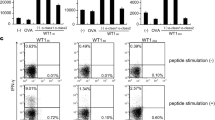
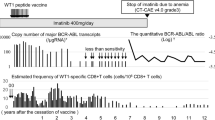
Simultaneous induction of tumor antigen-specific cytotoxic T lymphocytes (CTLs) and helper T lymphocytes (HTLs) is required for an optimal anti-tumor immune response. WT1332, a 16-mer WT1-derived helper peptide, induce HTLs in an HLA class II-restricted manner and enhance the induction of WT1-specific CTLs in vitro. However, in vivo immune reaction to WT1332 vaccination in tumor-bearing patients remained unclear. Here, a striking difference in WT1-specific T cell responses was shown between WT1 CTL + WT1 helper peptide and WT1 CTL peptide vaccines in patients with recurrent glioma. WT1-specific CTLs were more strongly induced in the patients who were immunized with WT1 CTL + WT1 helper peptide vaccine, compared to those who were immunized with WT1 CTL vaccine alone. Importantly, a clear correlation was demonstrated between WT1-specific CTL and WT1332-specific HTL responses. Interestingly, two novel distinct populations of WT1-tetramerlow WT1-TCRlow CD5low and WT1-tetramerhigh WT1-TCRhigh CD5high CTLs were dominantly detected in WT1 CTL + WT1 helper peptide vaccine. Although natural WT1 peptide-reactive CTLs in the latter population were evidently less than those in the former population, the latter population showed natural WT1 peptide-specific proliferation capacity comparable to the former population, suggesting that the latter population highly expressing CD5, a marker of resistance to activation-induced cell death, should strongly expand and persist for a long time in patients. These results demonstrated the advantage of WT1 helper peptide vaccine for the enhancement of WT1-specific CTL induction by WT1 CTL peptide vaccine.




Similar content being viewed by others
Abbreviations
- APC:
-
Allophycocyanin
- 7-AAD:
-
7-Amino-Actinomycin D
- FITC:
-
Fluorescein isothiocyanate
- HLA:
-
Human Leukocyte Antigen
- IL:
-
Interleukin
- IFN:
-
Interferon
- PE:
-
Phycoerythrin
- TCR:
-
T cell receptor
- TNF:
-
Tumor Necrosis Factor
- TAP:
-
Transporter associated with Antigen Processing
References
Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480–483. https://doi.org/10.1038/31002
Joffre OP, Segura E, Savina A, Amigorena S (2012) Cross-presentation by dendritic cells. Nat Rev Immunol 12:557–569. https://doi.org/10.1038/nri3254
Bos R, Sherman LA (2010) CD4 + T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8 + T lymphocytes. Cancer Res 70:8368–8377. https://doi.org/10.1158/0008-5472.CAN-10-1322
Nakata J, Nakajima H, Hayashibara H, Imafuku K, Morimoto S, Fujiki F, Motooka D, Okuzaki D, Hasegawa K, Hosen N, Tsuboi A, Oka Y, Kumanogoh A, Oji Y, Sugiyama H (2018) Extremely strong infiltration of WT1-specific CTLs into mouse tumor by the combination vaccine with WT1-specific CTL and helper peptides. Oncotarget. 9:36029–36038. https://doi.org/10.18632/oncotarget.26338
Ahrends T, Spanjaard A, Pilzecker B, Babala N, Bovens A, Xiao Y, Jacobs H, Borst J (2017) CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 47(848–61):e5. https://doi.org/10.1016/j.immuni.2017.10.009
Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R (2011) Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA 108:21182–21187. https://doi.org/10.1073/pnas.1118450109
Sun JC, Bevan MJ (2003) Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342. https://doi.org/10.1126/science.1083317
Sconocchia G, Eppenberger-Castori S, Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S, Spagnoli GC, Lauro D, Lugli A, Han J, Iezzi G, Ferrone C, Ferlosio A, Tornillo L, Droeser R, Rossi P, Attanasio A, Ferrone S, Terracciano L (2014) HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 16:31–42. https://doi.org/10.1593/neo.131568
Nasman A, Andersson E, Marklund L, Tertipis N, Hammarstedt-Nordenvall L, Attner P, Nyberg T, Masucci GV, Munck-Wikland E, Ramqvist T, Dalianis T (2013) HLA class I and II expression in oropharyngeal squamous cell carcinoma in relation to tumor HPV status and clinical outcome. PLoS ONE 8:e77025. https://doi.org/10.1371/journal.pone.0077025
Esteban F, Ruiz-Cabello F, Concha A, Perez-Ayala M, Sanchez-Rozas JA, Garrido F (1990) HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin Exp Metastasis 8:319–328
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 15:5323–5337
Fujiki F, Oka Y, Tsuboi A, Kawakami M, Kawakatsu M, Nakajima H, Elisseeva OA, Harada Y, Ito K, Li Z, Tatsumi N, Sakaguchi N, Fujioka T, Masuda T, Yasukawa M, Udaka K, Kawase I, Oji Y, Sugiyama H (2007) Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J Immunother 30:282–293. https://doi.org/10.1097/01.cji.0000211337.91513.94
Fujiki F, Oka Y, Kawakatsu M, Tsuboi A, Nakajima H, Elisseeva OA, Harada Y, Li Z, Tatsumi N, Kamino E, Shirakata T, Nishida S, Taniguchi Y, Kawase I, Oji Y, Sugiyama H (2008) A WT1 protein-derived, naturally processed 16-mer peptide, WT1(332), is a promiscuous helper peptide for induction of WT1-specific Th1-type CD4(+) T cells. Microbiol Immunol 52:591–600. https://doi.org/10.1111/j.1348-0421.2008.00080.x
Anguille S, Fujiki F, Smits EL, Oji Y, Lion E, Oka Y, Berneman ZN, Sugiyama H (2013) Identification of a Wilms’ tumor 1-derived immunogenic CD4(+) T-cell epitope that is recognized in the context of common Caucasian HLA-DR haplotypes. Leukemia 27:748–750. https://doi.org/10.1038/leu.2012.248
Kobayashi Y, Sakura T, Miyawaki S, Toga K, Sogo S, Heike Y (2017) A new peptide vaccine OCV-501: in vitro pharmacology and phase 1 study in patients with acute myeloid leukemia. Cancer Immunol Immunother 66:851–863. https://doi.org/10.1007/s00262-017-1981-3
Lin Y, Fujiki F, Katsuhara A, Oka Y, Tsuboi A, Aoyama N, Tanii S, Nakajima H, Tatsumi N, Morimoto S, Tamanaka T, Tachino S, Hosen N, Nishida S, Oji Y, Kumanogoh A, Sugiyama H (2013) HLA-DPB1*05: 01-restricted WT1332-specific TCR-transduced CD4 + T lymphocytes display a helper activity for WT1-specific CTL induction and a cytotoxicity against leukemia cells. J Immunother 36:159–170. https://doi.org/10.1097/CJI.0b013e3182873581
Fujiki F, Oka Y, Kawakatsu M, Tsuboi A, Tanaka-Harada Y, Hosen N, Nishida S, Shirakata T, Nakajima H, Tatsumi N, Hashimoto N, Taguchi T, Ueda S, Nonomura N, Takeda Y, Ito T, Myoui A, Izumoto S, Maruno M, Yoshimine T, Noguchi S, Okuyama A, Kawase I, Oji Y, Sugiyama H (2010) A clear correlation between WT1-specific Th response and clinical response in WT1 CTL epitope vaccination. Anticancer Res. 30:2247–2254
Tsuboi A, Hashimoto N, Fujiki F, Morimoto S, Kagawa N, Nakajima H, Hosen N, Nishida S, Nakata J, Morita S, Sakamoto J, Oji Y, Oka Y, Sugiyama H (2019) A phase I clinical study of a cocktail vaccine of Wilms’ tumor 1 (WT1) HLA class I and II peptides for recurrent malignant glioma. Cancer Immunol Immunother 68:331–340. https://doi.org/10.1007/s00262-018-2274-1
Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, White DE, Rosenberg SA (2003) Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother 26:349–356
Slingluff CL Jr, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, Whiteside TL, Leming PD, Kirkwood JM (2013) A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res 19:4228–4238. https://doi.org/10.1158/1078-0432.CCR-13-0002
Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, Hashimoto N, Maruno M, Elisseeva OA, Shirakata T, Kawakami M, Oji Y, Nishida S, Ohno S, Kawase I, Hatazawa J, Nakatsuka S, Aozasa K, Morita S, Sakamoto J, Sugiyama H, Yoshimine T (2008) Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 108:963–971. https://doi.org/10.3171/JNS/2008/108/5/0963
Tsuboi A, Oka Y, Udaka K, Murakami M, Masuda T, Nakano A, Nakajima H, Yasukawa M, Hiraki A, Oji Y, Kawakami M, Hosen N, Fujioka T, Wu F, Taniguchi Y, Nishida S, Asada M, Ogawa H, Kawase I, Sugiyama H (2002) Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother 51:614–620. https://doi.org/10.1007/s00262-002-0328-9
Nishida S, Ishikawa T, Egawa S, Koido S, Yanagimoto H, Ishii J, Kanno Y, Kokura S, Yasuda H, Oba MS, Sato M, Morimoto S, Fujiki F, Eguchi H, Nagano H, Kumanogoh A, Unno M, Kon M, Shimada H, Ito K, Homma S, Oka Y, Morita S, Sugiyama H (2018) Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: a phase II randomized study. Cancer Immunol Res. https://doi.org/10.1158/2326-6066.CIR-17-0386
Hashimoto N, Tsuboi A, Kagawa N, Chiba Y, Izumoto S, Kinoshita M, Kijima N, Oka Y, Morimoto S, Nakajima H, Morita S, Sakamoto J, Nishida S, Hosen N, Oji Y, Arita N, Yoshimine T, Sugiyama H (2015) Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 64:707–716. https://doi.org/10.1007/s00262-015-1674-8
Friedlein G, El Hage F, Vergnon I, Richon C, Saulnier P, Lecluse Y, Caignard A, Boumsell L, Bismuth G, Chouaib S, Mami-Chouaib F (2007) Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol. 178:6821–6827. https://doi.org/10.4049/jimmunol.178.11.6821
Tabbekh M, Franciszkiewicz K, Haouas H, Lecluse Y, Benihoud K, Raman C, Mami-Chouaib F (2011) Rescue of tumor-infiltrating lymphocytes from activation-induced cell death enhances the antitumor CTL response in CD5-deficient mice. J Immunol. 187:102–109. https://doi.org/10.4049/jimmunol.1004145
Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC (2015) The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol 16:107–117. https://doi.org/10.1038/ni.3043
Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW (2011) Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 29:2924–2932. https://doi.org/10.1200/JCO.2010.33.8053
Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ (2005) The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity 23:29–40. https://doi.org/10.1016/j.immuni.2005.05.009
Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W (2018) CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18:635–647. https://doi.org/10.1038/s41577-018-0044-0
Zanetti M (2015) Tapping CD4 T cells for cancer immunotherapy: the choice of personalized genomics. J Immunol. 194:2049–2056. https://doi.org/10.4049/jimmunol.1402669
Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A (1998) Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 160:3363–3373
Kobayashi H, Wood M, Song Y, Appella E, Celis E (2000) Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res 60:5228–5236
Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, Schadendorf D, Croockewit A, Blokx WA, Van Rossum MM, Kwok WW, Adema GJ, Punt CJ, Figdor CG (2013) Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 73:19–29. https://doi.org/10.1158/0008-5472.CAN-12-1127
Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, Uchiyama K, Kajihara M, Arakawa H, Misawa T, Toyama Y, Yanagisawa S, Ikegami M, Kan S, Hayashi K, Komita H, Kamata Y, Ito M, Ishidao T, Yusa S, Shimodaira S, Gong J, Sugiyama H, Ohkusa T, Tajiri H (2014) Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res 20:4228–4239. https://doi.org/10.1158/1078-0432.CCR-14-0314
Aloysius MM, Mc Kechnie AJ, Robins RA, Verma C, Eremin JM, Farzaneh F, Habib NA, Bhalla J, Hardwick NR, Satthaporn S, Sreenivasan T, El-Sheemy M, Eremin O (2009) Generation in vivo of peptide-specific cytotoxic T cells and presence of regulatory T cells during vaccination with hTERT (class I and II) peptide-pulsed DCs. J Transl Med. 7:18. https://doi.org/10.1186/1479-5876-7-18
Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM (2007) Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 178:1564–1572. https://doi.org/10.4049/jimmunol.178.3.1564
Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP (2005) Central memory self/tumor-reactive CD8 + T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA 102:9571–9576. https://doi.org/10.1073/pnas.0503726102
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550–4557. https://doi.org/10.1158/1078-0432.CCR-11-0116
Mandl JN, Monteiro JP, Vrisekoop N, Germain RN (2013) T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38:263–274. https://doi.org/10.1016/j.immuni.2012.09.011
Katsuhara A, Fujiki F, Aoyama N, Tanii S, Morimoto S, Oka Y, Tsuboi A, Nakajima H, Kondo K, Tatsumi N, Nakata J, Nakae Y, Takashima S, Nishida S, Hosen N, Sogo S, Oji Y, Sugiyama H (2015) Transduction of a novel HLA-DRB1*04:05-restricted, WT1-specific TCR gene into human CD4 + T cells confers killing activity against human leukemia cells. Anticancer Res 35:1251–1261
Precis
WT1 killer + helper peptide vaccine-induced greater WT1-specific CTL response than WT1 killer peptide vaccine in recurrent glioma patients, thereby revealing the existence of two distinct populations, WT1-tetramerhigh TCRhigh CD5high and WT1-tetramerlow TCRlow CD5low T-cells.
Author information
Authors and Affiliations
Contributions
FF and HS organized this study and prepared this manuscript. AK and NH recruited the patients, carried out the clinical investigations and collected patients’ clinical data. FF, SM, and MI performed the experiments. HN, JN, SN, KH, NH, YoO, and YuO contributed to the acquisition of the patient samples and conception of the study. SS supported this study and gave useful suggestions.
Corresponding author
Ethics declarations
Financial disclosure
Department of Cancer Immunology is a department in collaboration with Otsuka Pharmaceutical Co., Ltd. and is supported with a grant from the company. This study was, in part, supported by JSPS KAKENHI Grant Number JP17K07215.
Conflict of interest
Shinji Sogo is an employee of Otsuka Pharmaceutical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujiki, F., Tsuboi, A., Morimoto, S. et al. Identification of two distinct populations of WT1-specific cytotoxic T lymphocytes in co-vaccination of WT1 killer and helper peptides. Cancer Immunol Immunother 70, 253–263 (2021). https://doi.org/10.1007/s00262-020-02675-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02675-9