Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with an immune suppressive phenotype. They represent a critical component of the immune suppressive niche described in cancer, where they support immune escape and tumor progression through direct effects on both the innate and adaptive immune responses, largely by contributing to maintenance of a high oxidative stress environment. The number of MDSCs positively correlates with protumoral activity, and often diminishes the effectiveness of immunotherapies, which is particularly problematic with the emergence of personalized medicine. Approaches targeting MDSCs showed promising results in preclinical studies and are under active investigation in clinical trials in combination with various immune checkpoint inhibitors. In this review, we discuss MDSC targets and therapeutic approaches targeting MDSC that have the aim of enhancing the existing tumor therapies.
Similar content being viewed by others
Abbreviations
- Arg1:
-
Arginase 1
- ATRA:
-
All-trans retinoic acid
- BTK:
-
Bruton’s tyrosine kinase
- CCL:
-
C-C motif chemokine ligand
- CCR:
-
C-C motif chemokine receptor
- COX2:
-
Cyclooxygenase-2
- CXCL:
-
C-X-C motif chemokine
- CXCR:
-
C-X-C chemokine receptor
- FAO:
-
Fatty acid oxidation
- HADHA:
-
Mitochondrial trifunctional protein alpha subunit
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor-1α
- iNOS:
-
Inducible nitric oxide synthase
- M-MDSC:
-
Monocytic-myeloid-derived suppressor cell
- MIF:
-
Macrophage migration inhibitory factor
- mTOR:
-
Mammalian target of rapamycin
- MyD88:
-
Myeloid differentiation primary response gene 88
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- PDE5:
-
Phosphodiesterase-5
- PGE2:
-
Prostaglandin E2
- PMN-MDSC:
-
Polymorphonuclear-myeloid-derived suppressor cell
- ROS:
-
Reactive oxygen species
- Tregs:
-
Regulatory T cells
References
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. https://doi.org/10.1038/nri2506
Choi HS, Ha SY, Kim HM, Ahn SM, Kang MS, Kim KM, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, Kang ES (2016) The prognostic effects of tumor infiltrating regulatory T cells and myeloid derived suppressor cells assessed by multicolor flow cytometry in gastric cancer patients. Oncotarget 7(7):7940–7951. https://doi.org/10.18632/oncotarget.6958
Chevalier MF, Trabanelli S, Racle J, Salome B, Cesson V, Gharbi D, Bohner P, Domingos-Pereira S, Dartiguenave F, Fritschi AS, Speiser DE, Rentsch CA, Gfeller D, Jichlinski P, Nardelli-Haefliger D, Jandus C, Derre L (2017) ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J Clin Invest 127(8):2916–2929. https://doi.org/10.1172/JCI89717
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40(11):2969–2975. https://doi.org/10.1002/eji.201040895
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22(4):275–281. https://doi.org/10.1016/j.semcancer.2012.01.011
Bronte V, Zanovello P (2005) Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol 5(8):641–654. https://doi.org/10.1038/nri1668
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202(7):931–939. https://doi.org/10.1084/jem.20050715
Serafini P, Mgebroff S, Noonan K, Borrello I (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68(13):5439–5449. https://doi.org/10.1158/0008-5472.CAN-07-6621
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH (2006) Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66(2):1123–1131. https://doi.org/10.1158/0008-5472.CAN-05-1299
Kumar V, Patel S, Tcyganov E, Gabrilovich DI (2016) The Nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37(3):208–220. https://doi.org/10.1016/j.it.2016.01.004
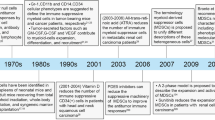
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13(10):739–752. https://doi.org/10.1038/nrc3581
Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ (2015) Myeloid-derived suppressor cells: paradoxical roles in infection and immunity. J Innate Immun 7(2):116–126. https://doi.org/10.1159/000368233
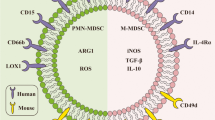
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150. https://doi.org/10.1038/ncomms12150
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22(2):238–244. https://doi.org/10.1016/j.coi.2010.01.021
Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI (2004) Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 172(2):989–999
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207(11):2439–2453. https://doi.org/10.1084/jem.20100587
Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V (2010) Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 40(1):22–35. https://doi.org/10.1002/eji.200939903
Jordan KR, Kapoor P, Spongberg E, Tobin RP, Gao D, Borges VF, McCarter MD (2017) Immunosuppressive myeloid-derived suppressor cells are increased in splenocytes from cancer patients. Cancer Immunol Immunother 66(4):503–513. https://doi.org/10.1007/s00262-016-1953-z
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 1319:47–65. https://doi.org/10.1111/nyas.12469
Haverkamp JM, Smith AM, Weinlich R, Dillon CP, Qualls JE, Neale G, Koss B, Kim Y, Bronte V, Herold MJ, Green DR, Opferman JT, Murray PJ (2014) Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity 41(6):947–959. https://doi.org/10.1016/j.immuni.2014.10.020
Pillay J, Tak T, Kamp VM, Koenderman L (2013) Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci 70(20):3813–3827. https://doi.org/10.1007/s00018-013-1286-4
Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL (2011) In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol 41(3):749–759. https://doi.org/10.1002/eji.201041069
Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 70(1):68–77. https://doi.org/10.1158/0008-5472.CAN-09-2587
Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182(9):5693–5701. https://doi.org/10.4049/jimmunol.0900092
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI (2007) Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 13(7):828–835. https://doi.org/10.1038/nm1609
Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ (2011) Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol 46(2):156–164. https://doi.org/10.3109/00365521.2010.516450
Li H, Han Y, Guo Q, Zhang M, Cao X (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 182(1):240–249
Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R (2012) Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 61(6):827–838. https://doi.org/10.1007/s00262-011-1143-y
Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanislawek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I (2016) Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res 76(11):3156–3165. https://doi.org/10.1158/0008-5472.CAN-15-2528
Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM (2001) Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol 166(9):5398–5406
Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, Gulen D, Bishay J, Talmadge JE (2009) Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol 9(7–8):937–948. https://doi.org/10.1016/j.intimp.2009.03.021
Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA (2010) Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 70(9):3526–3536. https://doi.org/10.1158/0008-5472.CAN-09-3278
Waight JD, Hu Q, Miller A, Liu S, Abrams SI (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 6(11):e27690. https://doi.org/10.1371/journal.pone.0027690
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+ Ly6C+ granulocytes. Proc Natl Acad Sci USA 107(50):21248–21255. https://doi.org/10.1073/pnas.1015855107
Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF (2007) Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 117(12):3846–3856. https://doi.org/10.1172/JCI31871
Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, Sytwu HK (2013) Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep 1(2):139–151. https://doi.org/10.1016/j.stemcr.2013.06.006 (eCollection 2013)
Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F (2010) Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120(2):457–471. https://doi.org/10.1172/JCI40483
Diao J, Yang X, Song X, Chen S, He Y, Wang Q, Chen G, Luo C, Wu X, Zhang Y (2015) Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol 32(2):453. https://doi.org/10.1007/s12032-014-0453-2
Hu X, Li B, Li X, Zhao X, Wan L, Lin G, Yu M, Wang J, Jiang X, Feng W, Qin Z, Yin B, Li Z (2014) Transmembrane TNF-alpha promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol 192(3):1320–1331. https://doi.org/10.4049/jimmunol.1203195
Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14(5):408–419. https://doi.org/10.1016/j.ccr.2008.10.011
Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V (2006) Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116(10):2777–2790. https://doi.org/10.1172/JCI28828
Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P (2003) IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 170(1):270–278
Munera V, Popovic PJ, Bryk J, Pribis J, Caba D, Matta BM, Zenati M, Ochoa JB (2010) Stat 6-dependent induction of myeloid derived suppressor cells after physical injury regulates nitric oxide response to endotoxin. Ann Surg 251(1):120–126. https://doi.org/10.1097/SLA.0b013e3181bfda1c
Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR (2010) Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116(25):5738–5747. https://doi.org/10.1182/blood-2010-06-287839
Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, Garrett WS (2015) CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid-derived suppressor cell population and function. Cell Rep 12(2):244–257. https://doi.org/10.1016/j.celrep.2015.06.024
Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP (2007) Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110(2):624–631. https://doi.org/10.1182/blood-2007-01-065714
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2(10):1096–1103
Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sultmann H, Altevogt P, Umansky V, Momma S (2015) Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. https://doi.org/10.1080/2162402X.2015.1008371
Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205(10):2235–2249. https://doi.org/10.1084/jem.20080132
Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 181(7):4666–4675
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211(5):781–790. https://doi.org/10.1084/jem.20131916
Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP (2011) Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 9(9):e1001162. https://doi.org/10.1371/journal.pbio.1001162
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+ CD11b+ myeloid cells that promote metastasis. Cancer Cell 13(1):23–35. https://doi.org/10.1016/j.ccr.2007.12.004
Umansky V, Blattner C, Gebhardt C, Utikal J (2017) CCR5 in recruitment and activation of myeloid-derived suppressor cells in melanoma. Cancer Immunol Immunother 66(8):1015–1023. https://doi.org/10.1007/s00262-017-1988-9
Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, Li F, Yang L, Huang J, Yang S, Li H, Zhao X, Dong W, Yan Y, Zhao S, Huang B, Zhang B, Zhang Y (2017) CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-beta-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 6(6):e1320011. https://doi.org/10.1080/2162402X.2017.1320011
Trabanelli S, Chevalier MF, Martinez-Usatorre A, Gomez-Cadena A, Salome B, Lecciso M, Salvestrini V, Verdeil G, Racle J, Papayannidis C, Morita H, Pizzitola I, Grandclement C, Bohner P, Bruni E, Girotra M, Pallavi R, Falvo P, Leibundgut EO, Baerlocher GM, Carlo-Stella C, Taurino D, Santoro A, Spinelli O, Rambaldi A, Giarin E, Basso G, Tresoldi C, Ciceri F, Gfeller D, Akdis CA, Mazzarella L, Minucci S, Pelicci PG, Marcenaro E, McKenzie ANJ, Vanhecke D, Coukos G, Mavilio D, Curti A, Derre L, Jandus C (2017) Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat Commun 8(1):593. https://doi.org/10.1038/s41467-017-00678-2
Younis RH, Han KL, Webb TJ (2016) Human head and neck squamous cell carcinoma-associated semaphorin 4D induces expansion of myeloid-derived suppressor cells. J Immunol 196(3):1419–1429. https://doi.org/10.4049/jimmunol.1501293
Wang T, Chu Z, Lin H, Jiang J, Zhou X, Liang X (2014) Galectin-3 contributes to cisplatin-induced myeloid derived suppressor cells (MDSCs) recruitment in Lewis lung cancer-bearing mice. Mol Biol Rep 41(6):4069–4076. https://doi.org/10.1007/s11033-014-3276-5
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809. https://doi.org/10.1038/nrc2734
Dufait I, Van Valckenborgh E, Menu E, Escors D, De Ridder M, Breckpot K (2016) Signal transducer and activator of transcription 3 in myeloid-derived suppressor cells: an opportunity for cancer therapy. Oncotarget 7(27):42698–42715. https://doi.org/10.18632/oncotarget.8311
Wu L, Yan C, Czader M, Foreman O, Blum JS, Kapur R, Du H (2012) Inhibition of PPARgamma in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood 119(1):115–126. https://doi.org/10.1182/blood-2011-06-363093
Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, Nguyen JM, Curiel TJ, Cadungog MG, Singhal S, Eruslanov EB, Zhang P, Tchou J, Zhang R, Conejo-Garcia JR (2017) Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov 7(1):72–85. https://doi.org/10.1158/2159-8290.CD-16-0502
Liu YF, Chen YY, He YY, Wang JY, Yang JP, Zhong SL, Jiang N, Zhou P, Jiang H, Zhou J (2017) Expansion and activation of granulocytic, myeloid-derived suppressor cells in childhood precursor B cell acute lymphoblastic leukemia. J Leukoc Biol 102(2):449–458. https://doi.org/10.1189/jlb.5MA1116-453RR
Wang SH, Lu QY, Guo YH, Song YY, Liu PJ, Wang YC (2016) The blockage of Notch signalling promoted the generation of polymorphonuclear myeloid-derived suppressor cells with lower immunosuppression. Eur J Cancer 68:90–105. https://doi.org/10.1016/j.ejca.2016.08.019
Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P (2011) MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 11(7):856–861. https://doi.org/10.1016/j.intimp.2011.01.030
Abad C, Nobuta H, Li J, Kasai A, Yong WH, Waschek JA (2014) Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J Leukoc Biol 95(2):357–367. https://doi.org/10.1189/jlb.1012531
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188(1):21–28. https://doi.org/10.4049/jimmunol.1101029
Sugimoto Y, Narumiya S (2007) Prostaglandin E receptors. J Biol Chem 282(16):11613–11617. https://doi.org/10.1074/jbc.R600038200
Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 67(9):4507–4513. https://doi.org/10.1158/0008-5472.CAN-06-4174
Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P (2011) Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118(20):5498–5505. https://doi.org/10.1182/blood-2011-07-365825
Inamoto S, Itatani Y, Yamamoto T, Minamiguchi S, Hirai H, Iwamoto M, Hasegawa S, Taketo MM, Sakai Y, Kawada K (2016) Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clin Cancer Res 22(2):492–501. https://doi.org/10.1158/1078-0432.CCR-15-0726
Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA (2016) Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov 6(1):80–95. https://doi.org/10.1158/2159-8290.CD-15-0224
Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, He J, Peng JY, Duan TH, Cui J, Zhang XS, Zhou FJ, Wang RF, Li J (2017) CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 36(15):2095–2104. https://doi.org/10.1038/onc.2016.367
Najjar YG, Rayman P, Jia X, Pavicic PG Jr, Rini BI, Tannenbaum C, Ko J, Haywood S, Cohen P, Hamilton T, Diaz-Montero CM, Finke J (2017) Myeloid-derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of IL1beta, IL8, CXCL5, and Mip-1alpha. Clin Cancer Res 23(9):2346–2355. https://doi.org/10.1158/1078-0432.CCR-15-1823
Kusmartsev S, Gabrilovich DI (2003) Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol 74(2):186–196
Greifenberg V, Ribechini E, Rossner S, Lutz MB (2009) Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol 39(10):2865–2876. https://doi.org/10.1002/eji.200939486
Kusmartsev S, Gabrilovich DI (2005) STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol 174(8):4880–4891
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111(8):4233–4244. https://doi.org/10.1182/blood-2007-07-099226
Lechner MG, Liebertz DJ, Epstein AL (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 185(4):2273–2284. https://doi.org/10.4049/jimmunol.1000901
Yaddanapudi K, Rendon BE, Lamont G, Kim EJ, Al Rayyan N, Richie J, Albeituni S, Waigel S, Wise A, Mitchell RA (2016) MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol Res 4(2):101–112. https://doi.org/10.1158/2326-6066.CIR-15-0070-T
Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123(Pt 10):1603–1611. https://doi.org/10.1242/jcs.064386
Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG (2009) Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124(11):2621–2633. https://doi.org/10.1002/ijc.24249
Pyzer AR, Stroopinsky D, Rajabi H, Washington A, Tagde A, Coll M, Fung J, Bryant MP, Cole L, Palmer K, Somaiya P, Karp Leaf R, Nahas M, Apel A, Jain S, McMasters M, Mendez L, Levine J, Joyce R, Arnason J, Pandolfi PP, Kufe D, Rosenblatt J, Avigan D (2017) MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood 129(13):1791–1801. https://doi.org/10.1182/blood-2016-07-730614
Liu Y, Munoz N, Tsai AC, Logan TM, Ma T (2017) Metabolic reconfiguration supports reacquisition of primitive phenotype in human mesenchymal stem cell aggregates. Stem Cells 35(2):398–410. https://doi.org/10.1002/stem.2510
Tsai AC, Liu Y, Yuan X, Ma T (2015) Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng Part A 21(9–10):1705–1719. https://doi.org/10.1089/ten.TEA.2014.0314
Liu Y, Ma T (2015) Metabolic regulation of mesenchymal stem cell in expansion and therapeutic application. Biotechnol Prog 31(2):468–481. https://doi.org/10.1002/btpr.2034
Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L (1995) A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 182(6):1683–1693
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F (2012) Role of monocarboxylate transporters in human cancers: state of the art. J Bioenergy Biomembr 44(1):127–139. https://doi.org/10.1007/s10863-012-9428-1
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sanchez-Garcia FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52. https://doi.org/10.3389/fimmu.2016.00052
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513(7519):559–563. https://doi.org/10.1038/nature13490
Bronte V (2014) Tumor cells hijack macrophages via lactic acid. Immunol Cell Biol 92(8):647–649. https://doi.org/10.1038/icb.2014.67
Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M (2012) Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biol 13:18. https://doi.org/10.1186/1471-2121-13-18
Jian SL, Chen WW, Su YC, Su YW, Chuang TH, Hsu SC, Huang LR (2017) Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis. Cell Death Dis 8(5):e2779. https://doi.org/10.1038/cddis.2017.192
Wu T, Zhao Y, Wang H, Li Y, Shao L, Wang R, Lu J, Yang Z, Wang J, Zhao Y (2016) mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci Rep 6:20250. https://doi.org/10.1038/srep20250
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC (2015) Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res 3(11):1236–1247. https://doi.org/10.1158/2326-6066.CIR-15-0036
Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC (2017) Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology 6(10):e1344804. https://doi.org/10.1080/2162402X.2017.1344804
Roda JM, Parihar R, Carson WE III (2005) CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol 175(3):1619–1627
Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C (2011) The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology 133(2):221–238. https://doi.org/10.1111/j.1365-2567.2011.03429.x
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63(15):4441–4449
Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI (2006) All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 66(18):9299–9307. https://doi.org/10.1158/0008-5472.CAN-06-1690
Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI (2007) Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 67(22):11021–11028. https://doi.org/10.1158/0008-5472.CAN-07-2593
Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D (2013) Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother 62(5):909–918. https://doi.org/10.1007/s00262-013-1396-8
Ipilimumab and all-trans retinoic acid combination treatment of stage IV melanoma. Identifier: NCT02403778 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02403778. Accessed 13 Mar 2018
Bill MA, Fuchs JR, Li C, Yui J, Bakan C, Benson DM Jr, Schwartz EB, Abdelhamid D, Lin J, Hoyt DG, Fossey SL, Young GS, Carson WE III, Li PK, Lesinski GB (2010) The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol Cancer 9:165. https://doi.org/10.1186/1476-4598-9-165
Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC, Yang CS (2012) Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 5(2):205–215. https://doi.org/10.1158/1940-6207.CAPR-11-0247
Kortylewski M, Moreira D (2017) Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities. Cancer Immunol Immunother 66(8):979–988. https://doi.org/10.1007/s00262-017-1966-2
Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, Jones J, D’Apuzzo M, Forman S, Kortylewski M (2015) TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin Cancer Res 21(16):3771–3782. https://doi.org/10.1158/1078-0432.CCR-14-3145
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 181(1):346–353
Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, Ghiringhelli F (2016) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res 76(18):5241–5252. https://doi.org/10.1158/0008-5472.CAN-15-3164
Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, Georgoulias V, Kotsakis A (2016) Effect of first-line treatment on myeloid-derived suppressor cells’ subpopulations in the peripheral blood of patients with non-small cell lung cancer. J Thorac Oncol 11(8):1263–1272. https://doi.org/10.1016/j.jtho.2016.04.026
Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP (2010) COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 10:464. https://doi.org/10.1186/1471-2407-10-464
Study of E7046 in subjects with selected advanced malignancies. Indentifier: NCT02540291 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02540291. Accessed 13 Mar 2018
Albu DI, Wang Z, Huang KC, Wu J, Twine N, Leacu S, Ingersoll C, Parent L, Lee W, Liu D, Wright-Michaud R, Kumar N, Kuznetsov G, Chen Q, Zheng W, Nomoto K, Woodall-Jappe M, Bao X (2017) EP4 antagonism by E7046 diminishes myeloid immunosuppression and synergizes with Treg-reducing IL-2-diphtheria toxin fusion protein in restoring anti-tumor immunity. Oncoimmunology 6(8):e1338239. https://doi.org/10.1080/2162402X.2017.1338239
Hong DS, Parikh A, Shapiro G, Varga A, Naing A, Meric-Bernstam F, Reyderman L, Bao X, Binder TA, Ren M, Siu A, Xu L, Liu M, Dayal S, Bhagawati-Prasad V, Tchakov I, Owa T, Ooi CE, Marabelle A (2018) Phase I study of E7046, a novel PGE2 receptor type 4 inhibitor, in patients with advanced solid tumors: Clinical results and effects on myeloid- and T-lymphoid cell-mediated immunosuppression. In: 2018 ASCO-SITC clinical immuno-oncology symposium. J Clin Oncol 36 (suppl 5S; Abstract 49)
Evans EE, Jonason AS Jr, Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, Kirk R, Howell A, Giralico S, Scrivens M, Klimatcheva K, Fisher TL, Bowers WJ, Paris M, Smith ES, Zauderer M (2015) Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunol Res 3(6):689–701. https://doi.org/10.1158/2326-6066.CIR-14-0171
Patnaik A, Weiss GJ, Leonard JE, Rasco DW, Sachdev JC, Fisher TL, Winter LA, Reilly C, Parker RB, Mutz D, Blaydorn L, Tolcher AW, Zauderer M, Ramanathan RK (2016) Safety, pharmacokinetics, and pharmacodynamics of a humanized anti-semaphorin 4D antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res 22(4):827–836. https://doi.org/10.1158/1078-0432.CCR-15-0431
VX15/2503 in combination with avelumab in advanced non-small cell lung cancer. Identifier: NCT03268057 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03268057. Accessed 13 Mar 2018
Anti-SEMA4D monoclonal antibody VX15/2503 with nivolumab or ipilimumab in treating patients with stage III or IV melanoma. Identifier: NCT03425461 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03425461. Accessed 13 Mar 2018
VX15/2503 and immunotherapy in resectable pancreatic and colorectal cancer. Identifier: NCT03373188 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT03373188. Accessed 13 Mar 2018
Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL (2014) Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med 6(237):237ra267. https://doi.org/10.1126/scitranslmed.3007974
Wang-Gillam A, Noel MS, Sleijfer S, Jung H, Lohr L, Zhao N, Miao S, Potarca A, Charo I, Bekker P, Schall TJ (2016) The inhibition of CCR2 to modify the microenvironment in pancreatic cancer mouse model and to support the profiling of the CCR2 inhibitor CCX872-B in patients. In: ASCO annual meeting 2016. J Clin Oncol 34 (suppl 15; Abstract e15743)
Linehan D, Noel MS, Hezel AF, Wang-Gillam A, Eskens F, Sleijfer S, Desar IME, Erdkamp F, Wilmink J, Diehl J, Potarca A, Zhao N, Miao S, Deng J, Hillson J, Bekker P, Schall TJ, Singh R (2018) Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. In: 2018 ASCO-SITC clinical immuno-oncology symposium. J Clin Oncol 36 (suppl 5S; Abstract 92)
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 203(12):2691–2702. https://doi.org/10.1084/jem.20061104
Wesolowski R, Markowitz J, Carson WE III (2013) Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J Immunother Cancer 1:10. https://doi.org/10.1186/2051-1426-1-10
Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, Halama N, Dimitrakopoulou-Strauss A, Haefeli WE, Jager D, Enk A, Utikal J, Umansky V (2017) Tadalafil has biologic activity in human melanoma. Results of a pilot trial with tadalafil in patients with metastatic melanoma (TaMe). Oncoimmunology 6(9):e1326440. https://doi.org/10.1080/2162402X.2017.1326440
De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, Musiani P, Zanovello P, Bronte V (2005) Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci USA 102(11):4185–4190. https://doi.org/10.1073/pnas.0409783102
Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, Li F, Chen X, Ping Y, Wang D, Gao Q, He Q, Huang L, Li H, Huang J, Zhao X, Xue W, Sun Z, Lu J, Yu JJ, Zhao J, Zhang B, Zhang Y (2018) Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res 78(7):1779–1791. https://doi.org/10.1158/0008-5472.CAN-17-2460
De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T (2016) Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 539(7629):443–447. https://doi.org/10.1038/nature20554
Dose-escalation a study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of IPI-549. Identifier: NCT02637531 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02637531. Accessed 13 Mar 2018
Pachter JA, Weaver DT (2018) Effect of dual PI3K-δ,γ inhibitor duvelisib on immunosuppressive Tregs and myeloid cells to enhance efficacy of checkpoint and co-stimulatory antibodies in a B cell lymphoma model. In: 2018 ASCO-SITC clinical immuno-oncology symposium. J Clin Oncol 36 (suppl 5S; Abstract 33)
Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005) Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11(18):6713–6721. https://doi.org/10.1158/1078-0432.CCR-05-0883
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70(8):3052–3061. https://doi.org/10.1158/0008-5472.CAN-09-3690
Rong Y, Yuan CH, Qu Z, Zhou H, Guan Q, Yang N, Leng XH, Bu L, Wu K, Wang FB (2016) Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep 6:23824. https://doi.org/10.1038/srep23824
Depletion of myeloid derived suppressor cells to enhance anti PD-1 therapy national library of medicine. https://clinicaltrials.gov/ct2/show/NCT03302247. Accessed 6 Dec 2017
Combination therapy for patients with untreated metastatic pancreatic ductal adenocarcinoma. Identifier: NCT02754726 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT02754726. Accessed 13 Mar 2018
First-line, gemcitabine, cisplatin + ipilimumab for metastatic urothelial carcinoma. Identifier: NCT01524991 ClinicalTrials.gov. https://ClinicalTrials.gov/show/NCT01524991. Accessed 13 Mar 2018
Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, Kaltcheva T, Leong L, Morgan R, Chung V, Tinsley R, Eng M, Wilczynski S, Ellenhorn JDI, Diamond DJ, Cristea M (2018) p53-reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin Cancer Res 24(6):1315–1325. https://doi.org/10.1158/1078-0432.CCR-17-2709
Kanterman J, Sade-Feldman M, Biton M, Ish-Shalom E, Lasry A, Goldshtein A, Hubert A, Baniyash M (2014) Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res 74(21):6022–6035. https://doi.org/10.1158/0008-5472.CAN-14-0657
Wang Z, Liu Y, Zhang Y, Shang Y, Gao Q (2016) MDSC-decreasing chemotherapy increases the efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma and pancreatic cancer. Oncotarget 7(4):4760–4769. https://doi.org/10.18632/oncotarget.6734
Isherwood J, Arshad A, Chung W, Runau F, Cooke J, Pollard C, Thompson J, Metcalfe M, Dennison A (2017) Parenteral omega 3 significantly reduces myeloid-derived suppressor cells in palliative pancreatic patients receiving gemcitabine and intravenous omega 3 compared to patients receiving gemcitabine only treatment. In: ESPEN congress 2017. Clin Nutr 36 (suppl; Abstract P107)
Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S (2015) Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res 75(13):2629–2640. https://doi.org/10.1158/0008-5472.CAN-14-2921
Heine A, Schilling J, Grunwald B, Kruger A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P, Diehl L, Hochst B (2016) The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol Immunother 65(3):273–282. https://doi.org/10.1007/s00262-015-1790-5
Myeloid derived suppressor cells and chronic myeloid leukemia national library of medicine. https://clinicaltrials.gov/ct2/show/record/NCT03214718. Accessed 11 Jul 2017
Stiff A, Trikha P, Wesolowski R, Kendra K, Hsu V, Uppati S, McMichael E, Duggan M, Campbell A, Keller K, Landi I, Zhong Y, Dubovsky J, Howard JH, Yu L, Harrington B, Old M, Reiff S, Mace T, Tridandapani S, Muthusamy N, Caligiuri MA, Byrd JC, Carson WE III (2016) Myeloid-derived suppressor cells express Bruton’s tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Res 76(8):2125–2136. https://doi.org/10.1158/0008-5472.CAN-15-1490
Seliger B, Giersberg C, Staehler MD (2011) Modulation of immune cell subpopulations in renal cell carcinoma patients by sunitinib. In: 2011 ASCO/ASTRO/SUO genitourinary cancers symposium. J Clin Oncol 29 (suppl 7: Abstract 395)
Martin del Campo SE, Levine KM, Mundy-Bosse BL, Grignol VP, Fairchild ET, Campbell AR, Trikha P, Mace TA, Paul BK, Jaime-Ramirez AC, Markowitz J, Kondadasula SV, Guenterberg KD, McClory S, Karpa VI, Pan X, Olencki TE, Monk JP, Mortazavi A, Tridandapani S, Lesinski GB, Byrd JC, Caligiuri MA, Shah MH, Carson WE III (2015) The Raf kinase inhibitor sorafenib inhibits JAK-STAT signal transduction in human immune cells. J Immunol 195(5):1995–2005. https://doi.org/10.4049/jimmunol.1400084
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, Gotz M, Guillaume P, Dohner H, Bunjes D, Schmitt M (2008) Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol 36(10):1297–1308. https://doi.org/10.1016/j.exphem.2008.05.002
Guo C, Hu F, Yi H, Feng Z, Li C, Shi L, Li Y, Liu H, Yu X, Wang H, Li J, Li Z, Wang XY (2016) Myeloid-derived suppressor cells have a proinflammatory role in the pathogenesis of autoimmune arthritis. Ann Rheum Dis 75(1):278–285. https://doi.org/10.1136/annrheumdis-2014-205508
Hurez V, Daniel BJ, Sun L, Liu AJ, Ludwig SM, Kious MJ, Thibodeaux SR, Pandeswara S, Murthy K, Livi CB, Wall S, Brumlik MJ, Shin T, Zhang B, Curiel TJ (2012) Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res 72(8):2089–2099. https://doi.org/10.1158/0008-5472.CAN-11-3019
Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83(1):64–70. https://doi.org/10.1189/jlb.0407247
Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI (2017) Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res 23(12):2942–2950. https://doi.org/10.1158/1078-0432.CCR-16-1784
Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, Roth J, Yi Q, Overwijk WW, Kwak LW (2014) Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20(6):676–681. https://doi.org/10.1038/nm.3560
Funding
We are grateful for the generous support from the Toni Stephenson Lymphoma Center at Beckman Research Institute of City of Hope. This work was also supported by the US Department of Defense Peer Reviewed Cancer Career Development Award (W81XWH-14-PRCRP-CDA) to HQ and a Quest for Cure grant from the Leukemia and Lymphoma Society (0855-14) to LWK.
Author information
Authors and Affiliations
Contributions
YL and DLS wrote and edited the manuscript; GW, WAC, ZD, HS, VYL, and S-CC participated in drafting the manuscript; HQ and LWK revised the manuscript critically.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Liu, Y., Wei, G., Cheng, W.A. et al. Targeting myeloid-derived suppressor cells for cancer immunotherapy. Cancer Immunol Immunother 67, 1181–1195 (2018). https://doi.org/10.1007/s00262-018-2175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2175-3