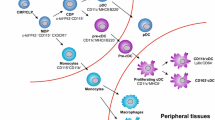
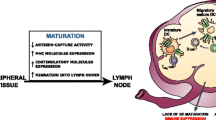
Abstract
Dendritic cells (DC) play unique and diverse roles in the tumor occurrence, development, progression and response to therapy. First of all, DC can actively uptake tumor-associated antigens, process them and present antigenic peptides to T cells inducing and maintaining tumor-specific T cell responses. DC interaction with different immune effector cells may also support innate antitumor immunity, as well as humoral responses also known to inhibit tumor development in certain cases. On the other hand, DC are recruited to the tumor site by specific tumor-derived and stroma-derived factors, which may also impair DC maturation, differentiation and function, thus resulting in the deficient formation of antitumor immune response or development of DC-mediated tolerance and immune suppression. Identification of DC-stimulating and DC-suppressing/polarizing factors in the tumor environment and the mechanism of DC modulation are important for designing effective DC-based vaccines and for recovery of immunodeficient resident DC responsible for maintenance of clinically relevant antitumor immunity in patients with cancer. DC-targeting tumor-derived factors and their effects on resident and administered DC in the tumor milieu are described and discussed in this review.

Similar content being viewed by others
Abbreviations
- ACVR1:
-
Activin receptor 1
- BMPR:
-
Bone morphogenetic protein receptor
- COX:
-
Cyclooxygenases
- CSF-1:
-
Colony-stimulating factor 1
- CSIF:
-
Cytokine synthesis inhibitory factor
- CTL:
-
Cytotoxic T cell(s)
- DAMP:
-
Damage-associated molecular pattern
- DC:
-
Dendritic cell(s)
- DR6:
-
Death receptor 6
- ER:
-
Endoplasmic reticulum
- FABP4:
-
Fatty acid-binding protein
- GDF-15:
-
Growth differentiation factor-15
- HCG:
-
Human chorionic gonadotropin
- HMGB1:
-
Chromatin-binding protein high-mobility group box 1
- HSP:
-
Heat-shock protein(s)
- IDO:
-
Indoleamine-2, 3-dioxygenase
- IL-10:
-
Interleukin-10
- LPL:
-
Lipoprotein lipase
- M-CSF:
-
Macrophage colony-stimulating factor
- MIC-1:
-
Macrophage inhibitory cytokine-1
- MMP:
-
Matrix metalloproteinase
- MUC1:
-
Mucin 1
- NMDAR:
-
N-methyl-d-aspartate receptor
- PD-L1:
-
Programmed death ligand 1
- PSA:
-
Prostate-specific antigen
- RAGE:
-
Receptor for advanced glycation end product
- regDC:
-
Regulatory dendritic cell(s)
- ROS:
-
Reactive oxygen species
- SDF-1:
-
Stromal cell-derived factor-1
- STAT3:
-
Signal transducers and activators of transcription 3
- TGF-β:
-
Transforming growth factor beta
- TGFBR:
-
TGF-β receptor
- TIM-3:
-
T cell immunoglobulin domain and mucin domain-3
- TLR:
-
Toll-like receptor
- Treg:
-
Regulatory T cell(s)
- TREM1:
-
Triggering receptor expressed on myeloid cell-1
- UPR:
-
Unfolded protein response
- VEGF:
-
Vascular endothelial growth factor
- XBP1:
-
X-box-binding protein 1
References
Zhong H, Shurin MR, Han B (2007) Optimizing dendritic cell-based immunotherapy for cancer. Expert Rev Vaccin 6(3):333–345. doi:10.1586/14760584.6.3.333
Ma Y, Shurin GV, Gutkin DW, Shurin MR (2012) Tumor associated regulatory dendritic cells. Semin Cancer Biol 22(4):298–306. doi:10.1016/j.semcancer.2012.02.010
Shurin GV, Ma Y, Shurin MR (2013) Immunosuppressive mechanisms of regulatory dendritic cells in cancer. Cancer Microenviron 6(2):159–167. doi:10.1007/s12307-013-0133-3
Shurin GV, Ouellette CE, Shurin MR (2012) Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother 61(2):223–230. doi:10.1007/s00262-011-1138-8
Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4(1):36–44. doi:10.7150/jca.5046
Shurin MR, Naiditch H, Zhong H, Shurin GV (2011) Regulatory dendritic cells: new targets for cancer immunotherapy. Cancer Biol Ther 11(11):988–992
Garg AD, Dudek-Peric AM, Romano E, Agostinis P (2015) Immunogenic cell death. Int J Dev Biol 59(1–3):131–140. doi:10.1387/ijdb.150061pa
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1):1–5. doi:10.1189/jlb.0306164
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D (2014) HMGB1 in health and disease. Mol Aspects Med 40:1–116. doi:10.1016/j.mam.2014.05.001
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2010) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388. doi:10.1146/annurev.immunol.021908.132603
Saenz R, Souza Cda S, Huang CT, Larsson M, Esener S, Messmer D (2010) HMGB1-derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine 28(47):7556–7562. doi:10.1016/j.vaccine.2010.08.054
Saenz R, Futalan D, Leutenez L, Eekhout F, Fecteau JF, Sundelius S, Sundqvist S, Larsson M, Hayashi T, Minev B, Carson D, Esener S, Messmer B, Messmer D (2014) TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J Transl Med 12:211. doi:10.1186/1479-5876-12-211
Saenz R, Messmer B, Futalan D, Tor Y, Larsson M, Daniels G, Esener S, Messmer D (2014) Activity of the HMGB1-derived immunostimulatory peptide Hp91 resides in the helical C-terminal portion and is enhanced by dimerization. Mol Immunol 57(2):191–199. doi:10.1016/j.molimm.2013.09.007
Demoulin S, Herfs M, Somja J, Roncarati P, Delvenne P, Hubert P (2015) HMGB1 secretion during cervical carcinogenesis promotes the acquisition of a tolerogenic functionality by plasmacytoid dendritic cells. Int J Cancer 137(2):345–358. doi:10.1002/ijc.29389
Chen B, Miller AL, Rebelatto M, Brewah Y, Rowe DC, Clarke L, Czapiga M, Rosenthal K, Imamichi T, Chen Y, Chang CS, Chowdhury PS, Naiman B, Wang Y, Yang D, Humbles AA, Herbst R, Sims GP (2015) S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS ONE 10(2):e0115828. doi:10.1371/journal.pone.0115828
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL (2013) Functions of S100 proteins. Curr Mol Med 13(1):24–57
Lee TH, Jang AS, Park JS, Kim TH, Choi YS, Shin HR, Park SW, Uh ST, Choi JS, Kim YH, Kim Y, Kim S, Chung IY, Jeong SH, Park CS (2013) Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann Allergy Asthma Immunol 111(4):268–275. doi:10.1016/j.anai.2013.06.028
Srikrishna G (2012) S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun 4(1):31–40. doi:10.1159/000330095
Engelkamp D, Schafer BW, Mattei MG, Erne P, Heizmann CW (1993) Six S100 genes are clustered on human chromosome 1q21: identification of two genes coding for the two previously unreported calcium-binding proteins S100D and S100E. Proc Natl Acad Sci USA 90(14):6547–6551
Zhu L, Kohda F, Nakahara T, Chiba T, Tsuji G, Hachisuka J, Ito T, Tu Y, Moroi Y, Uchi H, Furue M (2013) Aberrant expression of S100A6 and matrix metalloproteinase 9, but not S100A2, S100A4, and S100A7, is associated with epidermal carcinogenesis. J Dermatol Sci 72(3):311–319. doi:10.1016/j.jdermsci.2013.07.005
Basso D, Fogar P, Plebani M (2013) The S100A8/A9 complex reduces CTLA4 expression by immature myeloid cells: implications for pancreatic cancer-driven immunosuppression. Oncoimmunology 2(6):e24441. doi:10.4161/onci.24441
Harpio R, Einarsson R (2004) S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin Biochem 37(7):512–518. doi:10.1016/j.clinbiochem.2004.05.012
Wang T, Liang Y, Thakur A, Zhang S, Yang T, Chen T, Gao L, Chen M, Ren H (2015) Diagnostic significance of S100A2 and S100A6 levels in sera of patients with non-small cell lung cancer. Tumour Biol. doi:10.1007/s13277-015-4057-z
Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE (2011) S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 123(11):1216–1226. doi:10.1161/CIRCULATIONAHA.110.985523
Bruhn S, Fang Y, Barrenas F, Gustafsson M, Zhang H, Konstantinell A, Kronke A, Sonnichsen B, Bresnick A, Dulyaninova N, Wang H, Zhao Y, Klingelhofer J, Ambartsumian N, Beck MK, Nestor C, Bona E, Xiang Z, Benson M (2014) A generally applicable translational strategy identifies S100A4 as a candidate gene in allergy. Sci Transl Med 6(218):218ra4. doi:10.1126/scitranslmed.3007410
Ampie L, Choy W, Lamano JB, Fakurnejad S, Bloch O, Parsa AT (2015) Heat shock protein vaccines against glioblastoma: from bench to bedside. J Neurooncol 123(3):441–448. doi:10.1007/s11060-015-1837-7
Pawaria S, Binder RJ (2011) CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun 2:521. doi:10.1038/ncomms1524
Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, Issels RD (2001) The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol 31(5):1602–1609. doi:10.1002/1521-4141(200105)31:5<1602:AID-IMMU1602>3.0.CO;2-W
Binder RJ, Srivastava PK (2005) Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8 + T cells. Nat Immunol 6(6):593–599. doi:10.1038/ni1201
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. doi:10.1038/nm0603-669
Toi M, Matsumoto T, Bando H (2001) Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol 2(11):667–673. doi:10.1016/S1470-2045(01)00556-3
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2(10):1096–1103
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998) Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92(11):4150–4166
Shi Y, Yu P, Zeng D, Qian F, Lei X, Zhao Y, Tang B, Hao Y, Luo H, Chen J, Tan Y (2014) Suppression of vascular endothelial growth factor abrogates the immunosuppressive capability of murine gastric cancer cells and elicits antitumor immunity. FEBS J 281(17):3882–3893. doi:10.1111/febs.12923
Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G (2005) Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 68(2–3):276–284. doi:10.1159/000086784
Kim R, Emi M, Tanabe K, Arihiro K (2006) Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 66(11):5527–5536. doi:10.1158/0008-5472.CAN-05-4128
Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI (2007) Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res 13(16):4840–4848. doi:10.1158/1078-0432.CCR-07-0409
Wang H, Zhang L, Zhang S, Li Y (2015) Inhibition of vascular endothelial growth factor by small interfering RNA upregulates differentiation, maturation and function of dendritic cells. Exper Therap Med 9(1):120–124. doi:10.3892/etm.2014.2059
Seeger P, Musso T, Sozzani S (2015) The TGF-beta superfamily in dendritic cell biology. Cytokine Growth Fact Rev 26(6):647–657. doi:10.1016/j.cytogfr.2015.06.002
Yasmin N, Konradi S, Eisenwort G, Schichl YM, Seyerl M, Bauer T, Stockl J, Strobl H (2013) Beta-Catenin promotes the differentiation of epidermal Langerhans dendritic cells. J Invest Derm 133(5):1250–1259. doi:10.1038/jid.2012.481
Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D (2001) Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood 98(10):2992–2998
Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE (2010) TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol 185(6):3248–3255. doi:10.4049/jimmunol.1000981
Lievens D, Habets KL, Robertson AK, Laouar Y, Winkels H, Rademakers T, Beckers L, Wijnands E, Boon L, Mosaheb M, Ait-Oufella H, Mallat Z, Flavell RA, Rudling M, Binder CJ, Gerdes N, Biessen EA, Weber C, Daemen MJ, Kuiper J, Lutgens E (2013) Abrogated transforming growth factor beta receptor II (TGFbetaRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur Heart J 34(48):3717–3727. doi:10.1093/eurheartj/ehs106
Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J (2006) Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immun 176(9):5637–5643
Song S, Yuan P, Wu H, Chen J, Fu J, Li P, Lu J, Wei W (2014) Dendritic cells with an increased PD-L1 by TGF-beta induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int Immunopharmacol 20(1):117–123. doi:10.1016/j.intimp.2014.02.027
Zhou Z, Li W, Song Y, Wang L, Zhang K, Yang J, Zhang W, Su H, Zhang Y (2013) Growth differentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specific immune response. PLoS ONE 8(11):e78618. doi:10.1371/journal.pone.0078618
Smith DR, Kunkel SL, Burdick MD, Wilke CA, Orringer MB, Whyte RI, Strieter RM (1994) Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol 145(1):18–25
Gu ZJ, Costes V, Lu ZY, Zhang XG, Pitard V, Moreau JF, Bataille R, Wijdenes J, Rossi JF, Klein B (1996) Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop. Blood 88(10):3972–3986
Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS (2005) Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun 338(2):1126–1136. doi:10.1016/j.bbrc.2005.10.065
Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR (2004) Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res 10(21):7260–7269. doi:10.1158/1078-0432.CCR-04-0872
Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM (2004) Interleukin-10 suppression of myeloid cell activation: a continuing puzzle. Immunology 113(3):281–292. doi:10.1111/j.1365-2567.2004.01988.x
Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV (2002) Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer 101(1):61–68. doi:10.1002/ijc.10576
Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL (2006) Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metas Rev 25(3):333–356. doi:10.1007/s10555-006-9010-6
Oosterhoff D, Lougheed S, van de Ven R, Lindenberg J, van Cruijsen H, Hiddingh L, Kroon J, van den Eertwegh AJ, Hangalapura B, Scheper RJ, de Gruijl TD (2012) Tumor-mediated inhibition of human dendritic cell differentiation and function is consistently counteracted by combined p38 MAPK and STAT3 inhibition. Oncoimmunology 1(5):649–658. doi:10.4161/onci.20365
Schwarz AM, Banning-Eichenseer U, Seidel K, Mauz-Korholz C, Korholz D, Staege MS (2013) Impact of interleukin-10 on phenotype and gene expression during early monocyte differentiation into dendritic cells. Anticancer Res 33(11):4791–4798
Hirano T (1998) Interleukin 6 and its receptor: 10 years later. Int Rev Immunol 16(3–4):249–284. doi:10.3109/08830189809042997
Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM (2002) Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 100(1):230–237
Nishio H, Yaguchi T, Sugiyama J, Sumimoto H, Umezawa K, Iwata T, Susumu N, Fujii T, Kawamura N, Kobayashi A, Park J, Aoki D, Kawakami Y (2014) Immunosuppression through constitutively activated NF-kappaB signalling in human ovarian cancer and its reversal by an NF-kappaB inhibitor. Br J Cancer 110(12):2965–2974. doi:10.1038/bjc.2014.251
Chomarat P, Banchereau J, Davoust J, Palucka AK (2000) IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 1(6):510–514. doi:10.1038/82763
Alshamsan A (2012) Induction of tolerogenic dendritic cells by IL-6-secreting CT26 colon carcinoma. Immunopharmacol Immunotoxicol 34(3):465–469. doi:10.3109/08923973.2011.625034
Yang L, Wu Q, Xu L, Zhang W, Zhu Y, Liu H, Xu J, Gu J (2015) Increased expression of colony stimulating factor-1 is a predictor of poor prognosis in patients with clear-cell renal cell carcinoma. BMC Cancer 15:67. doi:10.1186/s12885-015-1076-5
Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW (2002) The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 7(2):147–162
Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY (1998) Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood 92(12):4778–4791
Lo AS, Gorak-Stolinska P, Bachy V, Ibrahim MA, Kemeny DM, Maher J (2007) Modulation of dendritic cell differentiation by colony-stimulating factor-1: role of phosphatidylinositol 3′-kinase and delayed caspase activation. J Leukoc Biol 82(6):1446–1454. doi:10.1189/jlb.0307142
Demoulin SA, Somja J, Duray A, Guenin S, Roncarati P, Delvenne PO, Herfs MF, Hubert PM (2015) Cervical (pre)neoplastic microenvironment promotes the emergence of tolerogenic dendritic cells via RANKL secretion. Oncoimmunology 4(6):e1008334. doi:10.1080/2162402X.2015.1008334
Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, Lebecque S (2007) Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol 178(5):2763–2769
Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, Ronchese F (2008) Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother 57(11):1665–1673. doi:10.1007/s00262-008-0487-4
Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ (2001) Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 7(12):1339–1346. doi:10.1038/nm1201-1339
Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16(11):2927–2931. doi:10.1158/1078-0432.CCR-09-2329
Hargadon KM, Bishop JD, Brandt JP, Hand ZC, Ararso YT, Forrest OA (2016) Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunol Cell Biol 94(1):24–38. doi:10.1038/icb.2015.58
Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR (2003) Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol 170(5):2026–2030. doi:10.1097/01.ju.0000091264.46134.b7
Aalamian-Matheis M, Chatta GS, Shurin MR, Huland E, Huland H, Shurin GV (2007) Inhibition of dendritic cell generation and function by serum from prostate cancer patients: correlation with serum-free PSA. Adv Exp Med Biol 601:173–182
Pillai K, Pourgholami MH, Chua TC, Morris DL (2015) MUC1 as a potential target in anticancer therapies. Am J Clin Oncol 38(1):108–118. doi:10.1097/COC.0b013e31828f5a07
Carlos CA, Dong HF, Howard OM, Oppenheim JJ, Hanisch FG, Finn OJ (2005) Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol 175(3):1628–1635
Rughetti A, Pellicciotta I, Biffoni M, Backstrom M, Link T, Bennet EP, Clausen H, Noll T, Hansson GC, Burchell JM, Frati L, Taylor-Papadimitriou J, Nuti M (2005) Recombinant tumor-associated MUC1 glycoprotein impairs the differentiation and function of dendritic cells. J Immunol 174(12):7764–7772
Ueno A, Cho S, Cheng L, Wang J, Hou S, Nakano H, Santamaria P, Yang Y (2007) Transient upregulation of indoleamine 2,3-dioxygenase in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes 56(6):1686–1693. doi:10.2337/db06-1727
Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA (2000) Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol 164(7):3596–3599
Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P (2015) The role of gangliosides in neurodevelopment. Nutrients 7(5):3891–3913. doi:10.3390/nu7053891
Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM Jr (2001) Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res 61(1):363–369
Peguet-Navarro J, Sportouch M, Popa I, Berthier O, Schmitt D, Portoukalian J (2003) Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol 170(7):3488–3494
Bennaceur K, Popa I, Chapman JA, Migdal C, Peguet-Navarro J, Touraine JL, Portoukalian J (2009) Different mechanisms are involved in apoptosis induced by melanoma gangliosides on human monocyte-derived dendritic cells. Glycobiology 19(6):576–582. doi:10.1093/glycob/cwp015
Pugh S, Thomas GA (1994) Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut 35(5):675–678
Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD (2002) Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol 168(9):4333–4343
Trabanelli S, Lecciso M, Salvestrini V, Cavo M, Ocadlikova D, Lemoli RM, Curti A (2015) PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J Immunol Res 2015:253191. doi:10.1155/2015/253191
Schipper RG, Romijn JC, Cuijpers VM, Verhofstad AA (2003) Polyamines and prostatic cancer. Biochem Soc Trans 31(2):375–380. doi:10.1042/bst0310375
Erbas H, Bal O, Cakir E (2015) Effect of rosuvastatin on arginase enzyme activity and polyamine production in experimental breast cancer. Balkan Med J 32(1):89–95. doi:10.5152/balkanmedj.2015.15611
Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML (2003) Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 89(8):1463–1472. doi:10.1038/sj.bjc.6601243
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123(9):3685–3692. doi:10.1172/JCI69741
Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M (2006) Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107(5):2013–2021. doi:10.1182/blood-2005-05-1795
Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavolgyi E, Catrina AI, Wheelock CE, Vivar N, Rethi B (2013) Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol 191(6):3090–3099. doi:10.4049/jimmunol.1300772
Vaupel P, Mayer A (2016) Hypoxia-driven adenosine accumulation: a crucial microenvironmental factor promoting tumor progression. Adv Exp Med Biol 876:177–183. doi:10.1007/978-1-4939-3023-4_22
Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, Sun D (2015) A2B adenosine receptor activation switches differentiation of bone marrow cells to a CD11c(+)Gr-1(+) dendritic cell subset that promotes the Th17 response. Immun Inflamm Dis 3(4):360–373. doi:10.1002/iid3.74
Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112(5):1822–1831. doi:10.1182/blood-2008-02-136325
Ring S, Pushkarevskaya A, Schild H, Probst HC, Jendrossek V, Wirsdorfer F, Ledent C, Robson SC, Enk AH, Mahnke K (2015) Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J Immunol 194(8):3735–3744. doi:10.4049/jimmunol.1401434
Maroof A, English NR, Bedford PA, Gabrilovich DI, Knight SC (2005) Developing dendritic cells become ‘lacy’ cells packed with fat and glycogen. Immunology 115(4):473–483. doi:10.1111/j.1365-2567.2005.02181.x
Dong H, Bullock TN (2014) Metabolic influences that regulate dendritic cell function in tumors. Front Immunol 5:24. doi:10.3389/fimmu.2014.00024
Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16(8):880–886. doi:10.1038/nm.2172
Tyurin VA, Cao W, Tyurina YY, Gabrilovich DI, Kagan VE (2011) Mass-spectrometric characterization of peroxidized and hydrolyzed lipids in plasma and dendritic cells of tumor-bearing animals. Biochem Biophys Res Com 413(1):149–153. doi:10.1016/j.bbrc.2011.08.074
Gardner JK, Mamotte CD, Patel P, Yeoh TL, Jackaman C, Nelson DJ (2015) Mesothelioma tumor cells modulate dendritic cell lipid content, phenotype and function. PLoS ONE 10(4):e0123563. doi:10.1371/journal.pone.0123563
Gao F, Liu C, Guo J, Sun W, Xian L, Bai D, Liu H, Cheng Y, Li B, Cui J, Zhang C, Cai J (2015) Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer. Sci Rep 5:9613. doi:10.1038/srep09613
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH (2015) ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161(7):1527–1538. doi:10.1016/j.cell.2015.05.025
Wu R, Zhang QH, Lu YJ, Ren K, Yi GH (2015) Involvement of the IRE1alpha-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases. DNA Cell Biol 34(1):6–18. doi:10.1089/dna.2014.2552
Scanlon CS, Banerjee R, Inglehart RC, Liu M, Russo N, Hariharan A, van Tubergen EA, Corson SL, Asangani IA, Mistretta CM, Chinnaiyan AM, D’Silva NJ (2015) Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun 6:6885. doi:10.1038/ncomms7885
Covenas R, Munoz M (2014) Cancer progression and substance P. Histol Histopathol 29(7):881–890
Voedisch S, Rochlitzer S, Veres TZ, Spies E, Braun A (2012) Neuropeptides control the dynamic behavior of airway mucosal dendritic cells. PLoS ONE 7(9):e45951. doi:10.1371/journal.pone.0045951
Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, Amano H, Kurihara Y, Kurihara H, Okamoto H, Hoka S, Majima M (2008) Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci USA 105(36):13550–13555. doi:10.1073/pnas.0800767105
Gilaberte Y, Roca MJ, Garcia-Prats MD, Coscojuela C, Arbues MD, Vera-Alvarez JJ (2012) Neuropeptide Y expression in cutaneous melanoma. J Am Acad Dermatol 66(6):e201–e208. doi:10.1016/j.jaad.2011.02.015
Buttari B, Profumo E, Domenici G, Tagliani A, Ippoliti F, Bonini S, Businaro R, Elenkov I, Rigano R (2014) Neuropeptide Y induces potent migration of human immature dendritic cells and promotes a Th2 polarization. FASEB J 28(7):3038–3049. doi:10.1096/fj.13-243485
Makarenkova VP, Shurin GV, Tourkova IL, Balkir L, Pirtskhalaishvili G, Perez L, Gerein V, Siegfried JM, Shurin MR (2003) Lung cancer-derived bombesin-like peptides down-regulate the generation and function of human dendritic cells. J Neuroimmunol 145(1–2):55–67
DeRosa DC, Ryan PJ, Okragly A, Witcher DR, Benschop RJ (2008) Tumor-derived death receptor 6 modulates dendritic cell development. Cancer Immunol Immunother 57(6):777–787. doi:10.1007/s00262-007-0413-1
D’Souza-Schorey C, Clancy JW (2012) Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev 26(12):1287–1299. doi:10.1101/gad.192351.112
Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L (2006) Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res 66(18):9290–9298. doi:10.1158/0008-5472.CAN-06-1819
Huang SH, Li Y, Zhang J, Rong J, Ye S (2013) Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest 31(5):330–335. doi:10.3109/07357907.2013.789905
Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, Xiang J, Wu Z, Jiang G, Cao L (2015) Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget 6(30):29877–29888. doi:10.18632/oncotarget.4924
Feijoo E, Alfaro C, Mazzolini G, Serra P, Penuelas I, Arina A, Huarte E, Tirapu I, Palencia B, Murillo O, Ruiz J, Sangro B, Richter JA, Prieto J, Melero I (2005) Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer 116(2):275–281. doi:10.1002/ijc.21046
Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM (2003) Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest 111(5):727–735. doi:10.1172/JCI16492
Ristich V, Liang S, Zhang W, Wu J, Horuzsko A (2005) Tolerization of dendritic cells by HLA-G. Eur J Immunol 35(4):1133–1142. doi:10.1002/eji.200425741
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH), Grant RO1 CA154369 (to Shurin).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This paper is a Focussed Research Review based on a presentation given at the Fourth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2015), held in Ljubljana, Slovenia, 27th–30th April 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Zong, J., Keskinov, A.A., Shurin, G.V. et al. Tumor-derived factors modulating dendritic cell function. Cancer Immunol Immunother 65, 821–833 (2016). https://doi.org/10.1007/s00262-016-1820-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1820-y