Abstract
Introduction
Methods to induce T cell responses to protein vaccines have not been optimized. The immunostimulant AS15 has been administered with the recombinant MAGE-A3 protein (recMAGE-A3) i.m. but not i.d. or s.c. This study tests hypotheses that the i.d./s.c. route is safe and will increase CD4+ and CD8+ T cell responses to MAGE-A3.
Patients and methods
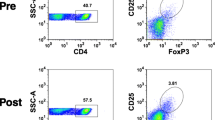
Twenty-five patients with resected stage IIB-IV MAGE-A3+ melanoma were randomized to immunization with recMAGE-A3 combined with AS15 immunostimulant (MAGE-A3 immunotherapeutic) either i.m. (group A, n = 13) or i.d./s.c. (group B, n = 12). Adverse events were recorded. Ab responses to MAGE-A3 were measured by ELISA. T cell responses to overlapping MAGE-A3 peptides were assessed in PBMC and a sentinel immunized node (SIN) after 1 in vitro stimulation with recMAGE-A3, by IFN-γ ELISPOT assay and by flow cytometry for multifunctional (TNF-α/IFN-γ) responses.
Results
Both routes of immunization were well tolerated without treatment-related grade 3 adverse events. All patients had durable Ab responses. For all 25 patients, the T cell response rate by ELISPOT assay was 30 % in SIN (7/23) but only 4 % (1/25) in PBMC. By flow cytometry, multifunctional CD8+ T cell responses were identified in one patient in each group; multifunctional CD4+ T cell response rates for groups A and B, respectively, were 31 and 64 % in SIN and 31 and 50 % in PBMC.
Conclusion
The MAGE-A3 immunotherapeutic was well tolerated after i.d./s.c. administration, with trends to higher CD4+ T cell response rates than with i.m. administration. This study supports further study of AS15 by i.d./s.c. administration.



Similar content being viewed by others
Abbreviations
- EBV-B:
-
Epstein–Barr virus-transformed B cells
- FFPE:
-
Formalin-fixed and paraffin-embedded
- GSK:
-
Glaxo Smith Kline
- IFA:
-
Incomplete Freund’s adjuvant
- MAGE-A3:
-
Immunotherapeutic recombinant MAGE-A3 combined with AS15 immunostimulant
- MPL:
-
Monophosphoryl lipid A
- recMAGE-A3:
-
Recombinant MAGE-A3 protein
- SIN:
-
Sentinel immunized node
- Tc99m-sc:
-
Technetium 99m-sulfur colloid
- TLR:
-
Toll-like receptor
References
Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S (2008) Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA 105(5):1650–1655
Burny W, Dantinne C, Amrani N, Vantomme V, Coulie P, Brichard V, Van Mechelen M (2006) Measurement of the specific T cell response in melanoma patients immunized with a recombinant MAGE-A3 immunotherapeutic: development of a sensitive read-out for serial monitoring. J Immunother 29(6):659–660 (Abstract)
Pellegrino P, Clementi E, Radice S (2015) On vaccine’s adjuvants and autoimmunity: current evidence and future perspectives. Autoimmun Rev 14(10):880–888
Olafsdottir T, Lindqvist M, Harandi AM (2015) Molecular signatures of vaccine adjuvants. Vaccine. doi:10.1016/j.vaccine.2015.04.099
Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, Maio M, Testori A, Dorval T, Grob JJ, Becker JC, Spatz A, Eggermont AM, Louahed J, Lehmann FF, Brichard VG, Keilholz U (2013) Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 31(19):2413–2420
Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, Voss G, Koutsoukos M, Pedneault L, Vandepapeliere P, McElrath MJ, Spearman P, Fuchs JD, Koblin BA, Blattner WA, Frey S, Baden LR, Harro C, Evans T (2007) Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 25(3):510–518
Sheikh NA, Petrylak D, Kantoff PW, dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL (2013) Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother 62(1):137–147
Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P (2005) Rapid and strong human CD8 + T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 115(3):739–746
Miconnet I, Koenig S, Speiser D, Krieg A, Guillaume P, Cerottini JC, Romero P (2002) CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol 168(3):1212–1218
Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA (2003) Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol 171(8):4320–4328
den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE (2005) CD4+ T cells are able to promote tumor growth through inhibition of tumor-specific CD8+ T-cell responses in tumor-bearing hosts. Cancer Res 65(15):6984–6989
Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM (2007) The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine 25(41):7145–7152
Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 6(8):769–776
Macleod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, Marrack P (2011) Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci USA 108(19):7914–7919
Kensil CR, Wu JY, Anderson CA, Wheeler DA, Amsden J (1998) QS-21 and QS-7: purified saponin adjuvants. Dev Biol Stand 92:41–47
Meraldi V, Romero JF, Kensil C, Corradin G (2005) A strong CD8+ T cell response is elicited using the synthetic polypeptide from the C-terminus of the circumsporozoite protein of Plasmodium berghei together with the adjuvant QS-21: quantitative and phenotypic comparison with the vaccine model of irradiated sporozoites. Vaccine 23(21):2801–2812
Schaed SG, Klimek VM, Panageas KS, Musselli CM, Butterworth L, Hwu WJ, Livingston PO, Williams L, Lewis JJ, Houghton AN, Chapman PB (2002) T-cell responses against tyrosinase 368–376 (370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund’s adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res 8(5):967–972
Vandepapeliere P, Horsmans Y, Moris P, Van MM, Janssens M, Koutsoukos M, Van BP, Clement F, Hanon E, Wettendorff M, Garcon N, Leroux-Roels G (2008) Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 26(10):1375–1386
Garcon N, Silvano J, Kuper CF, Baudson N, Gerard C, Forster R, Segal L (2015) Non-clinical safety evaluation of repeated intramuscular administration of the AS15 immunostimulant combined with various antigens in rabbits and cynomolgus monkeys. J Appl Toxicol. doi:10.1002/jat.3167
Destexhe E, Stannard D, Wilby OK, Grosdidier E, Baudson N, Forster R, Gerard CM, Garcon N, Segal L (2015) Nonclinical reproductive and developmental safety evaluation of the MAGE-A3 Cancer Immunotherapeutic, a therapeutic vaccine for cancer treatment. Reprod Toxicol 51:90–105
Destexhe E, Grosdidier E, Baudson N, Forster R, Gerard C, Garcon N, Segal L (2015) Non-clinical safety evaluation of single and repeated intramuscular administrations of MAGE-A3 Cancer Immunotherapeutic in rabbits and cynomolgus monkeys. J Appl Toxicol 35(7):717–728
van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254(5038):1643–1647
Tyagi P, Mirakhur B (2009) MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer 10(5):371–374
GlaxoSmithKline (2013) The investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoint in Phase III melanoma clinical trial. Retrieved from: http://www.gsk.com/en-gb/media/press-releases/2013/the-investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoint-in-phase-iii-melanoma-clinical-trial/. Accessed 10 Sept 2015
GlaxoSmithKline (2014) Investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoints in MAGRIT, a phase III non-small cell lung cancer clinical trial [Press release]. Retrieved from http://www.gsk.com/en-gb/media/press-releases/2014/investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoints-in-magrit-a-phase-iii-non-small-cell-lung-cancer-clinical-trial/. Accessed 10 Sept 2015
Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H (2008) Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity 29(3):497–510
Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I (2003) Epidermal Langerhans cell migration and sensitisation to chemical allergens. APMIS 111(7–8):797–804
Slingluff CL Jr, Yamshchikov GV, Hogan KT, Hibbitts SC, Petroni GR, Bissonette EA, Patterson JW, Neese PY, Grosh WW, Chianese-Bullock KA, Czarkowski A, Rehm PK, Parekh J (2008) Evaluation of the sentinel immunized node for immune monitoring of cancer vaccines. Ann Surg Oncol 15(12):3538–3549
Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, Patterson JW, Fink R, Rehm PK (2007) Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res 13(21):6386–6395
Gruselle O, Coche T, Louahed J (2015) Development of a quantitative real-time RT-PCR assay for the detection of MAGE-A3-positive tumors. J Mol Diagn 17(4):382–391
Vantomme V, Dantinne C, Amrani N, Permanne P, Gheysen D, Bruck C, Stoter G, Britten CM, Keilholz U, Lamers CH, Marchand M, Delire M, Gueguen M (2004) Immunologic analysis of a phase I/II study of vaccination with MAGE-3 protein combined with the AS02B adjuvant in patients with MAGE-3-positive tumors. J Immunother 27(2):124–135
Slingluff CL Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, Grosh WW, Boisvert ME, Kirkwood JM, Chianese-Bullock KA (2009) Effect of GM-CSF on circulating CD8+ and CD4+ T cell responses to a multipeptide melanoma vaccine: Outcome of a multicenter randomized trial. Clin Cancer Res 15(22):7036–7044
Crowder MJ, Hand DJ (1990) Analysis of repeated measures. CRC Press, Boca Raton, FL
Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW (2011) A randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 29(21):2924–2932
Koup RA, Douek DC (2011) Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb Perspect Med 1(1):a007252. doi:10.1101/cshperspect.a007252
Reed CM, Cresce ND, Mauldin IS, Slingluff CL Jr, Olson WC (2015) Vaccination with melanoma helper peptides induces antibody responses associated with improved overall survival. Clin Cancer Res 21(17):3879–3887
Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, Hensley ML, Konner J, Tew W, Spriggs DR, Hoffman EW, Venhaus R, Pan L, Salazar AM, Diefenbach CM, Old LJ, Gnjatic S (2012) Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 18(23):6497–6508
Slingluff CL Jr, Petroni GR, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, Bissonette EA, Barnd DL, Deacon DH, Patterson JW, Parekh J, Neese PY, Woodson EM, Wiernasz CJ, Merrill P (2004) Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol 22(22):4474–4485
Slingluff CL Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH (2003) Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol 21(21):4016–4026
Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Teates D, Neese P, Grosh WW, Petroni GR, Engelhard VH, Slingluff CL Jr (2001) Evaluation of peptide vaccine immunogenicity in draining lymph nodes and blood of melanoma patients. Int J Cancer 92(5):703–711
Stanton AW, Mellor RH, Cook GJ, Svensson WE, Peters AM, Levick JR, Mortimer PS (2003) Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat Res Biol 1(2):121–132
Brautigam P, Vanscheidt W, Foldi E, Krause T, Moser E (1993) The importance of the subfascial lymphatics in the diagnosis of lower limb edema: investigations with semiquantitative lymphoscintigraphy. Angiology 44(6):464–470
Slingluff CL Jr, Petroni GR, Olson W, Czarkowski AR, Grosh WW, Smolkin M, Chianese-Bullock KA, Neese PY, Deacon DH, Nail CJ, Merrill P, Fink R, Rehm PK (2008) Helper T cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol 26(30):4973–4980
Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL Jr (2013) Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother 62(7):1149–1159
Acknowledgments
This study was funded by GlaxoSmithKline Biologicals SA. Support was also provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579: Clinical Trials Office, Biorepository and Tissue Research Facility, Flow Cytometry Core, and Biomolecular Core Facility), Rebecca Clary Harris Memorial fellowship (to Ileana Mauldin) and T32 CA009109 fellowship (to Ileana Mauldin). Additional philanthropic support was provided by George S. Suddock. The Beirne Carter Center for Immunology Research provided flow cytometry support. We appreciate the work of Patrice Neese, Carmel Nail and Kathleen Haden for administering vaccines and for recording and managing toxicities. Appreciation also goes to clinical research coordinators Emily Allred, and Chris Blackwell, and to Cheryl Murphy Chase for preparing Epstein–Barr virus-transformed B cell lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Slingluff has the following relationships directly related to this work: The recombinant MAGE-A3 protein, overlapping peptides and AS15 were provided to the University of Virginia for this trial by GlaxoSmithKline, and the trial was funded largely by a grant to the University of Virginia from GlaxoSmithKline. The remaining authors have no conflicts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Slingluff, C.L., Petroni, G.R., Olson, W.C. et al. A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites. Cancer Immunol Immunother 65, 25–36 (2016). https://doi.org/10.1007/s00262-015-1770-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1770-9