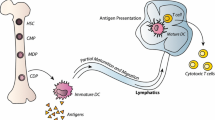
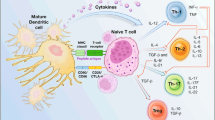
Abstract
Different approaches have been explored to raise effective antitumor responses against glioblastoma (GBM), the deadliest of primary brain tumors. In many clinical studies, cancer vaccines have been based on dendritic cells (DCs) loaded with peptides, representing one or more specific tumor antigens or whole lysates as a source of multiple antigens. Randomized clinical trials using DCs are ongoing, and results of efficacy are not yet available. Such strategies are feasible and safe; however, immune-suppressive microenvironment, absence of appropriate specific epitopes to target, and cancer immunoediting can limit their efficacy. The aim of this review is to describe how the definition of novel and more specific targets may increase considerably the possibility of successful DC immunotherapy. By proposing to target glioblastoma stem-like cells (GSCs), the immune response will be pointed to eradicating factors and pathways highly relevant to GBM biology. Preclinical observations on efficacy, and preliminary results of immunotherapy trials, encourage exploring the clinical efficacy of DC immunotherapy in GBM patients using high-purity, GSC-loaded DC vaccines.



Similar content being viewed by others
Abbreviations
- b-FGF:
-
Basic-fibroblast growth factor
- CSCs:
-
Cancer stem cells
- CUSA:
-
Cavitation ultrasonics surgical aspirator
- DCs:
-
Dendritic cells
- EGF:
-
Epidermal growth factor
- GBM:
-
Glioblastoma
- GMP:
-
Good manufacturing practice
- GSCs:
-
Glioblastoma stem-like cells
- HIF-1α:
-
Hypoxia-inducible factor-1α
- IFN-γ:
-
Interferon-γ
- NK:
-
Natural killer
- NS:
-
Neurospheres
- OS:
-
Overall survival
- PBMCs:
-
Peripheral blood mononuclear cells
- PFS:
-
Progression-free survival
- TAA:
-
Tumor-associated antigen
- TGF-β:
-
Transforming growth factor
- TMZ:
-
Temozolomide
- TNF-α:
-
Tumor necrosis factor-alpha
- VEGF:
-
Vascular endothelial growth factor
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331:1565–1570. doi:10.1126/science.1203486
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915. doi:10.1038/nm1100
Siesjö P, Visse E, Sjögren HO (1996) Cure of established, intracerebral rat gliomas induced by therapeutic immunizations with tumor cells and purified APC or adjuvant IFN-gamma treatment. J Immunother Emphas Tumor Immunol 19:334–345
Liau LM, Black KL, Prins RM et al (1999) Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg 90:1115–1124. doi:10.3171/jns.1999.90.6.1115
Witham TF, Erff ML, Okada H et al (2002) 7-Hydroxystaurosporine-induced apoptosis in 9L glioma cells provides an effective antigen source for dendritic cells and yields a potent vaccine strategy in an intracranial glioma model. Neurosurgery 50(6):1327–1335
Akasaki Y, Kikuchi T, Homma S et al (2001) Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother 24:106–113
Heimberger AB, Crotty LE, Archer GE et al (2000) Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol 103:16–25
Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M (2002) A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res 22:613–621
Kikuchi T, Akasaki Y, Abe T, Ohno T (2002) Intratumoral injection of dendritic and irradiated glioma cells induces anti-tumor effects in a mouse brain tumor model. Cancer Immunol Immunother 51:424–430
Ni HT, Spellman SR, Jean WC et al (2001) Immunization with dendritic cells pulsed with tumor extract increases survival of mice bearing intracranial gliomas. J Neurooncol 51:1–9
Okada H, Tahara H, Shurin MR et al (1998) Bone marrow-derived dendritic cells pulsed with a tumor-specific peptide elicit effective anti-tumor immunity against intracranial neoplasms. Int J Cancer 78:196–201
Pellegatta S, Finocchiaro G (2005) Cell therapies in neuro-oncology. Neurol Sci 26(Suppl 1):S43–S45. doi:10.1007/s10072-005-0405-x
Pellegatta S, Poliani PL, Corno D et al (2006) Dendritic cells pulsed with glioma lysates induce immunity against syngeneic intracranial gliomas and increase survival of tumor-bearing mice. Neurol Res 28:527–531. doi:10.1179/016164106X116809
De Vleeschouwer S, Van Calenbergh F, Demaerel P et al (2004) Transient local response and persistent tumor control in a child with recurrent malignant glioma: treatment with combination therapy including dendritic cell therapy. Case report. J Neurosurg 100:492–497
Rutkowski S, De Vleeschouwer S, Kaempgen E et al (2004) Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 91:1656–1662
Wheeler CJ, Das A, Liu G et al (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 10:5316–5326
Yamanaka R, Abe T, Yajima N et al (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179
Yamanaka R, Homma J, Yajima N et al (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167. doi:10.1158/1078-0432.CCR-05-0120
Yu JS, Liu G, Ying H et al (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979. doi:10.1158/0008-5472.CAN-03-3505
Yamanaka R, Homma J, Yajima N et al (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167. doi:10.1158/1078-0432.CCR-05-0120
Liau LM, Prins RM, Kiertscher SM et al (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525. doi:10.1158/1078-0432.CCR-05-0464
Polyzoidis S, Ashkan K (2014) Dendritic cell immunotherapy for glioblastoma. Expert Rev Anticancer Ther 14:761–763. doi:10.1586/14737140.2014.921571
Anguille S, Smits EL, Lion E et al (2014) Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15:e257–e267. doi:10.1016/S1470-2045(13)70585-0
Finocchiaro G, Pellegatta S (2014) Perspectives for immunotherapy in glioblastoma treatment. Curr Opin Oncol 26(6):608–614. doi:10.1097/CCO.0000000000000135
Lasky JL, Panosyan EH, Plant A et al (2013) Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res 33:2047–2056
Pellegatta S, Eoli M, Frigerio S et al (2013) The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology 2:e23401. doi:10.4161/onci.23401
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8:59–73. doi:10.1038/nri2216
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11:215–233. doi:10.1038/nrd3626
Sampson JH, Aldape KD, Archer GE et al (2011) Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol 13:324–333. doi:10.1093/neuonc/noq157
Eoli M, Pellegatta S, Frigerio S et al (2014) Association of increased progression-free survival in primary glioblastomas with lymphopenia at baseline and activation of NK and NKT cells after dendritic cell immunotherapy. In: ASCO Annual Meeting. J Clin Oncol 32:5 (suppl; abstr 2087)
Pellegatta S, Eoli M, Cantini G et al (2014) P02.03 * Increased count of NK and NKT cells are associated with prolonged survival in primary glioblastoma patients treated with dendritic cell immunotherapy in combination with radio- and chemo-therapy. Neuro Oncol 16:ii33. doi:10.1093/neuonc/nou174.119 (poster)
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. doi:10.1038/nature03128
Quintana E, Shackleton M, Sabel MS et al (2008) Efficient tumour formation by single human melanoma cells. Nature 456:593–598. doi:10.1038/nature07567
Beier D, Hau P, Proescholdt M et al (2007) CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67:4010–4015. doi:10.1158/0008-5472.CAN-06-4180
Ben-Porath I, Thomson MW, Carey VJ et al (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40:499–507. doi:10.1038/ng.127
Chen R, Nishimura MC, Bumbaca SM et al (2010) A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 17:362–375. doi:10.1016/j.ccr.2009.12.049
Pellegatta S, Poliani PL, Corno D et al (2006) Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res 66:10247–10252. doi:10.1158/0008-5472.CAN-06-2048
Ghods AJ, Irvin D, Liu G et al (2007) Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells 25:1645–1653. doi:10.1634/stemcells.2006-0624
Singh SK, Clarke ID, Terasaki M et al (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Tunici P, Bissola L, Lualdi E et al (2004) Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol Cancer 3:25. doi:10.1186/1476-4598-3-25
Lee J, Kotliarova S, Kotliarov Y et al (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403. doi:10.1016/j.ccr.2006.03.030
Verhaak RGW, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Soeda A, Park M, Lee D et al (2009) Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene 28:3949–3959. doi:10.1038/onc.2009.252
Bhat KPL, Balasubramaniyan V, Vaillant B et al (2013) Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. doi:10.1016/j.ccr.2013.08.001
Sottoriva A, Spiteri I, Piccirillo SGM et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014. doi:10.1073/pnas.1219747110
Bonavia R, Inda M-M, Cavenee WK, Furnari FB (2011) Heterogeneity maintenance in glioblastoma: a social network. Cancer Res 71:4055–4060. doi:10.1158/0008-5472.CAN-11-0153
Reardon DA (2015) Wen PY (2015) Glioma in 2014: unravelling tumour heterogeneity-implications for therapy. Nat Rev Clin Oncol 12(2):69–70. doi:10.1038/nrclinonc.2014.223
Stieber D, Golebiewska A, Evers L et al (2014) Glioblastomas are composed of genetically divergent clones with distinct tumourigenic potential and variable stem cell-associated phenotypes. Acta Neuropathol 127:203–219. doi:10.1007/s00401-013-1196-4
Di Tomaso T, Mazzoleni S, Wang E et al (2010) Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 16:800–813. doi:10.1158/1078-0432.CCR-09-2730
Wei J, Barr J, Kong L-Y et al (2010) Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther 9:67–78. doi:10.1158/1535-7163.MCT-09-0734
Wei J, Wu A, Kong L-Y et al (2011) Hypoxia potentiates glioma-mediated immunosuppression. PLoS One 6:e16195. doi:10.1371/journal.pone.0016195
Wu A, Wei J, Kong L-Y et al (2010) Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol 12:1113–1125. doi:10.1093/neuonc/noq082
Alizadeh D, Zhang L, Brown CE et al (2010) Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy. Clin Cancer Res 16:3399–3408. doi:10.1158/1078-0432.CCR-09-3087
Castriconi R, Daga A, Dondero A et al (2009) NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol 182:3530–3539. doi:10.4049/jimmunol.0802845
Brown CE, Starr R, Martinez C et al (2009) Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res 69:8886–8893. doi:10.1158/0008-5472.CAN-09-2687
Pallini R, Ricci-Vitiani L, Banna GL et al (2008) Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res 14:8205–8212. doi:10.1158/1078-0432.CCR-08-0644
De Bacco F, Casanova E, Medico E et al (2012) The MET oncogene is a functional marker of a glioblastoma stem cell subtype. Cancer Res 72:4537–4550. doi:10.1158/0008-5472.CAN-11-3490
Sampson JH, Heimberger AB, Archer GE et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. doi:10.1200/JCO.2010.28.6963
Xu Q, Liu G, Yuan X et al (2009) Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 27:1734–1740. doi:10.1002/stem.102
De Rosa A, Pellegatta S, Rossi M et al (2012) A radial glia gene marker, fatty acid binding protein 7 (FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS One 7:e52113. doi:10.1371/journal.pone.0052113
Cantini G, Pisati F, Pessina S et al (2012) Immunotherapy against the radial glia marker GLAST effectively triggers specific antitumor effectors without autoimmunity. Oncoimmunology 1:884–893. doi:10.4161/onci.20637
Favaro R, Appolloni I, Pellegatta S et al (2014) SOX2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendroglioma. Cancer Res 74:1833–1844. doi:10.1158/0008-5472.CAN-13-1942
Park D, Xiang AP, Mao FF et al (2010) Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 28:2162–2171. doi:10.1002/stem.541
Mehta S, Huillard E, Kesari S et al (2011) The central nervous system-restricted transcription factor Olig2 opposes p53 responses to genotoxic damage in neural progenitors and malignant glioma. Cancer Cell 19:359–371. doi:10.1016/j.ccr.2011.01.035
Vik-Mo EO, Nyakas M, Mikkelsen BV et al (2013) Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 62:1499–1509. doi:10.1007/s00262-013-1453-3
Ignatova TN, Kukekov VG, Laywell ED et al (2002) Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206. doi:10.1002/glia.10094
Phuphanich S, Wheeler CJ, Rudnick JD et al (2013) Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 62:125–135. doi:10.1007/s00262-012-1319-0
Orzan F, Pellegatta S, Poliani PL et al (2011) Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol Appl Neurobiol 37:381–394. doi:10.1111/j.1365-2990.2010.01132.x
Speranza MC, Frattini V, Pisati F et al (2012) NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Abstract 3:723–734
Frattini V, Pisati F, Speranza MC et al (2012) FOXP3, a novel glioblastoma proliferation and migration affects. Abstract 3:1146–1157
Patanè M, Porrati P, Bottega E et al (2013) Frequency of NFKBIA deletions is low in glioblastomas and skewed in glioblastoma neurospheres. Mol Cancer 12:160. doi:10.1186/1476-4598-12-160
Nava S, Dossena M, Pogliani S et al (2012) An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS One 7:e52301. doi:10.1371/journal.pone.0052301
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Kim H, Zheng S, Amini SS et al (2015) Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res 25(3):316–327. doi:10.1101/gr.180612.114
Acknowledgments
We thank the colleagues from the Department of Neurosurgery of the Istituto Besta, Dr Bianca Pollo and the colleagues of the Unit of Neuropathology, the staff of the cell factory (Cell Therapy Production Unit—UPTC), the Besta Brain Tumor Biobank (BBTB), Mr Piero Tieni (SOL Group Spa, Italy) for the cryo-management service and the technical assistance, Dr Lucia Cuppini for clinical data-management, and Drs Paola Porrati and Elisa Bottega for help in developing the GLP protocol for GSCs generation from CUSA bags. We thank the patients participating in the clinical studies and their families. DENDR-STEM clinical study is supported by Ministry of Health to G. Finocchiaro (RF-2010-2316156), and AIRC (Associazione Italiana per la ricerca sul cancro) (IG 2012 13043) to G. Finocchiaro. DENDR1 and DENDR2 are sponsored by Istituto Besta. DENDR1 is a study carried out as part of an oncology network (Rete Oncologica Lombarda) and funded referring to the deliberations of the regional council of Regione Lombardia no VIII/010761 of 11-12-2009 and DGR IX/1485 of 30-03-2011. Our preclinical studies have been supported by AIRC to S. Pellegatta (IG 2013 N. 14323), “il Fondo di Gio” and “Associazione Italiana Tumori Cerebrali” (AITC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Part of our data were reported in the abstract book of the conference “11th Congress of the European Association of Neuro-Oncology, Turin, Italy, October 9–12, 2014.”
Rights and permissions
About this article
Cite this article
Finocchiaro, G., Pellegatta, S. Immunotherapy with dendritic cells loaded with glioblastoma stem cells: from preclinical to clinical studies. Cancer Immunol Immunother 65, 101–109 (2016). https://doi.org/10.1007/s00262-015-1754-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1754-9