Abstract
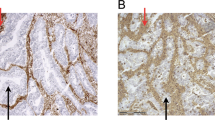
Besides being expressed on professional antigen-presenting cells, HLA class II antigens are expressed on various tumors of non-lymphoid origin, including a subset of colorectal cancers (CRC). Information about the regulation of HLA class II antigen expression is important for a better understanding of their role in the interactions between tumor and immune cells. Whether lack of HLA class II antigen expression in tumors reflects the selective immune destruction of HLA class II antigen-expressing tumor cells is unknown. To address this question, we tested whether lack of HLA class II antigen expression in CRC was associated with immune cell infiltration. We selected microsatellite-unstable (MSI-H) CRC, because they show pronounced tumor antigen-specific immune responses and, in a subset of tumors, lack of HLA class II antigen expression due to mutations inactivating HLA class II-regulatory genes. We examined HLA class II antigen expression, mutations in regulatory genes, and CD4-positive T cell infiltration in 69 MSI-H CRC lesions. Mutations in RFX5, CIITA, and RFXAP were found in 13 (28.9 %), 3 (6.7 %), and 1 (2.2 %) out of 45 HLA class II antigen-negative tumors. CD4-positive tumor-infiltrating lymphocyte counts were significantly higher in HLA class II antigen-negative tumors harboring mutations in HLA class II-regulatory genes (107.4 T cells per 0.25 mm2) compared to tumors without mutations (55.5 T cells per 0.25 mm2, p = 0.008). Our results suggest that the outgrowth of tumor cells lacking HLA class II antigen expression due to mutations of regulatory genes is favored in an environment of dense CD4-positive T cell infiltration.



Similar content being viewed by others
Abbreviations
- cMS:
-
Coding microsatellite
- CRC:
-
Colorectal cancer
- HE:
-
Hematoxylin and eosin
- HLA:
-
Human leukocyte antigen
- HNPCC:
-
Hereditary nonpolyposis colorectal cancer
- IHC:
-
Immunohistochemistry
- mAb:
-
Monoclonal antibody
- MSI-H:
-
High-level microsatellite instability
- PCR:
-
Polymerase chain reaction
References
Campoli M, Ferrone S (2008) HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene 27:5869–5885. doi:10.1038/onc.2008.273
Ferrone S, Campoli M (2006) A fresh look at an old story: revisiting HLA class II antigen expression by melanoma cells. Expert Rev Dermatol 1:805–823. doi:10.1586/17469872.1.6.805
Gutierrez J, Lopez-Nevot MA, Cabrera T, Oliva R, Esquivias J, Ruiz-Cabello F, Garrido F (1987) Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp Clin Immunogenet 4:144–152
Cabrera T, Ruiz-Cabello F, Garrido F (1995) Biological implications of HLA-DR expression in tumours. Scand J Immunol 41:398–406
Lovig T, Andersen SN, Thorstensen L, Diep CB, Meling GI, Lothe RA, Rognum TO (2002) Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer 87:756–762. doi:10.1038/sj.bjc.6600507
Morita M, Tanaka K, Kawanishi H, Tsuji M, Ookusa T, Takada H, Okamura A, Hioki K (1995) Immunohistochemically demonstrated expression of HLA-DR antigen in colorectal adenocarcinomas and its relation to clinicopathological features. J Surg Oncol 59:233–238. doi:10.1002/jso.2930590407
Walsh MD, Dent OF, Young JP et al (2009) HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer 125:1231–1237. doi:10.1002/ijc.24484
LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W (2004) Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol 34:1513–1525. doi:10.1002/eji.200424964
Michel S, Linnebacher M, Alcaniz J et al (2010) Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer 127:889–898. doi:10.1002/ijc.25106
Satoh A, Toyota M, Ikeda H et al (2004) Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-gamma-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene 23:8876–8886. doi:10.1038/sj.onc.1208144
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138(2073–87):e3. doi:10.1053/j.gastro.2009.12.064
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363:558–561. doi:10.1038/363558a0
Thibodeau SN, Bren G, Schaid D (1993) Microsatellite instability in cancer of the proximal colon. Science 260:816–819. doi:10.1126/science.8484122
Herman JG, Umar A, Polyak K et al (1998) Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 95:6870–6875. doi:10.1073/pnas.95.12.6870
Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M (2008) Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 134:988–997. doi:10.1053/j.gastro.2008.01.015
Kloor M, Michel S, von Knebel Doeberitz M (2010) Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer 127:1001–1010. doi:10.1002/ijc.25283
Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M (2001) Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 93:6–11. doi:10.1002/ijc.1298
Saeterdal I, Bjorheim J, Lislerud K et al (2001) Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA 98:13255–13260. doi:10.1073/pnas.231326898
Dolcetti R, Viel A, Doglioni C et al (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154:1805–1813. doi:10.1016/S0002-9440(10)65436-3
Buckowitz A, Knaebel HP, Benner A, Blaker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M (2005) Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer 92:1746–1753. doi:10.1038/sj.bjc.6602534
Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF (2006) Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark 2:69–86
Woerner SM, Yuan YP, Benner A, Korff S, von Knebel Doeberitz M, Bork P (2010) SelTarbase, a database of human mononucleotide-microsatellite mutations and their potential impact to tumorigenesis and immunology. Nucleic Acids Res 38:D682–D689. doi:10.1093/nar/gkp839
Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P (1996) Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol 6:1695–1697
Kloor M, Michel S, Buckowitz B et al (2007) Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer 121:454–458. doi:10.1002/ijc.22691
Kloor M, von Knebel Doeberitz M, Gebert JF (2005) Molecular testing for microsatellite instability and its value in tumor characterization. Expert Rev Mol Diagn 5:599–611. doi:10.1586/14737159.5.4.599
Boland CR, Thibodeau SN, Hamilton SR et al (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Findeisen P, Kloor M, Merx S et al (2005) T25 repeat in the 3′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res 65:8072–8078. doi:10.1158/0008-5472.CAN-04-4146
Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S (1993) Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods 161:239–256. doi:10.1016/0022-1759(93)90300-V
Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S (1989) Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma 8:85–95. doi:10.1089/hyb.1989.8.85
Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA 96:5215–5220
Wood GS, Warner NL, Warnke RA (1983) Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol 131:212–216
Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M (2008) High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 99:1867–1873. doi:10.1038/sj.bjc.6604756
Finke JH, Rayman P, Alexander J, Edinger M, Tubbs RR, Connelly R, Pontes E, Bukowski R (1990) Characterization of the cytolytic activity of CD4+ and CD8+ tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Res 50:2363–2370
Quezada SA, Simpson TR, Peggs KS et al (2010) Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 207:637–650. doi:10.1084/jem.20091918
Williams NS, Engelhard VH (1996) Identification of a population of CD4 + CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol 156:153–159
Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA (2010) Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 207:651–667. doi:10.1084/jem.20091921
Al-Batran SE, Rafiyan MR, Atmaca A et al (2005) Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res 65:3937–3941. doi:10.1158/0008-5472.CAN-04-4621
de Miranda NF, Goudkade D, Jordanova ES, Tops CM, Hes FJ, Vasen HF, van Wezel T, Morreau H (2012) Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res 18:1237–1245. doi:10.1158/1078-0432.CCR-11-1997
Acknowledgments
We are grateful to Nina Nelius, Beate Kuchenbuch and Petra Höfler for the excellent technical support they provided. This work was supported by the Public Health Service (PHS) Grants R01 CA110249 and R01 CA138188 (Soldano Ferrone), awarded by the National Cancer Institute and by a Grant of the Deutsche Forschungsgemeinschaft (German Research Foundation).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eva-Maria Surmann and Anita Y. Voigt have contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Surmann, EM., Voigt, A.Y., Michel, S. et al. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol Immunother 64, 357–366 (2015). https://doi.org/10.1007/s00262-014-1638-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1638-4