Abstract
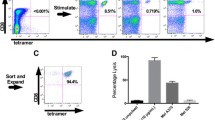
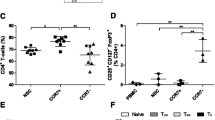
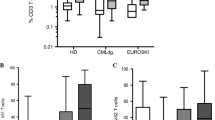
Gamma delta T cells (GDTc) comprise a small subset of cytolytic T cells shown to kill malignant cells in vitro and in vivo. We have developed a novel protocol to expand GDTc from human blood whereby GDTc were initially expanded in the presence of alpha beta T cells (ABTc) that were then depleted prior to use. We achieved clinically relevant expansions of up to 18,485-fold total GDTc, with 18,849-fold expansion of the Vδ1 GDTc subset over 21 days. ABTc depletion yielded 88.1 ± 4.2 % GDTc purity, and GDTc continued to expand after separation. Immunophenotyping revealed that expanded GDTc were mostly CD27-CD45RA- and CD27-CD45RA+ effector memory cells. GDTc cytotoxicity against PC-3M prostate cancer, U87 glioblastoma and EM-2 leukemia cells was confirmed. Both expanded Vδ1 and Vδ2 GDTc were cytotoxic to PC-3M in a T cell antigen receptor- and CD18-dependent manner. We are the first to label GDTc with ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles for cellular MRI. Using protamine sulfate and magnetofection, we achieved up to 40 % labeling with clinically approved Feraheme (Ferumoxytol), as determined by enumeration of Perls’ Prussian blue-stained cytospins. Electron microscopy at 2,800× magnification verified the presence of internalized clusters of iron oxide; however, high iron uptake correlated negatively with cell viability. We found improved USPIO uptake later in culture. MRI of GDTc in agarose phantoms was performed at 3 Tesla. The signal-to-noise ratios for unlabeled and labeled cells were 56 and 21, respectively. Thus, Feraheme-labeled GDTc could be readily detected in vitro via MRI.





Similar content being viewed by others
References
Hayday AC (2000) [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 18:975–1026
Kabelitz D, Wesch D, He W (2007) Perspectives of gammadelta T cells in tumor immunology. Cancer Res 67:5–8
Lamb LS Jr, Lopez RD (2005) gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant 11:161–168
Ensslin AS, Formby B (1991) Comparison of cytolytic and proliferative activities of human gamma delta and alpha beta T cells from peripheral blood against various human tumor cell lines. J Natl Cancer Inst 83:1564–1569
Zheng BJ, Chan KW, Im S, Chua D, Sham JS et al (2001) Anti-tumor effects of human peripheral gammadelta T cells in a mouse tumor model. Int J Cancer 92:421–425
Viey E, Lucas C, Romagne F, Escudier B, Chouaib S et al (2008) Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vgamma9Vdelta2 T cells from renal cell carcinoma patients. J Immunother 31:313–323
Knight A, Mackinnon S, Lowdell MW (2012) Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 14:1110–1118
Wright A, Lee JE, Link MP, Smith SD, Carroll W et al (1989) Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor delta chain. J Exp Med 169:1557–1564
Freedman MS, D’Souza S, Antel JP (1997) gamma delta T-cell-human glial cell interactions. I. In vitro induction of gammadelta T-cell expansion by human glial cells. J Neuroimmunol 74:135–142
Vollenweider I, Vrbka E, Fierz W, Groscurth P (1993) Heterogeneous binding and killing behaviour of human gamma/delta-TCR+ lymphokine-activated killer cells against K562 and Daudi cells. Cancer Immunol Immunother 36:331–336
Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP et al (2000) Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 96:384–392
Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y et al (2011) Human Vdelta1 gammadelta T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 13:753–764
Siegers GM, Felizardo TC, Mathieson AM, Kosaka Y, Wang XH et al (2011) Anti-leukemia activity of in vitro-expanded human gamma delta T cells in a xenogeneic Ph+ leukemia model. PLoS ONE 6:e16700
Gioia C, Agrati C, Casetti R, Cairo C, Borsellino G et al (2002) Lack of CD27-CD45RA-V gamma 9V delta 2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol 168:1484–1489
Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N et al (2003) Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med 198:391–397
Liu Z, Guo BL, Gehrs BC, Nan L, Lopez RD (2005) Ex vivo expanded human Vgamma9Vdelta2+ gammadelta-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J Urol 173:1552–1556
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S et al (2007) Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67:7450–7457
Yamaguchi T, Fujimiya Y, Suzuki Y, Katakura R, Ebina T (1997) A simple method for the propagation and purification of gamma delta T cells from the peripheral blood of glioblastoma patients using solid-phase anti-CD3 antibody and soluble IL-2. J Immunol Methods 205:19–28
Yamaguchi T, Suzuki Y, Katakura R, Ebina T, Yokoyama J et al (1998) Interleukin-15 effectively potentiates the in vitro tumor-specific activity and proliferation of peripheral blood gammadeltaT cells isolated from glioblastoma patients. Cancer Immunol Immunother 47:97–103
Fujimiya Y, Suzuki Y, Katakura R, Miyagi T, Yamaguchi T et al (1997) In vitro interleukin 12 activation of peripheral blood CD3(+)CD56(+) and CD3(+)CD56(-) gammadelta T cells from glioblastoma patients. Clin Cancer Res 3:633–643
Lamb LS Jr (2009) Gammadelta T cells as immune effectors against high-grade gliomas. Immunol Res 45:85–95
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H et al (2007) Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 56:469–476
Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K (2010) Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res 30:575–579
Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T et al (2010) A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg 37:1191–1197
Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I et al (2008) Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 57:1599–1609
Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K et al (2011) Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer 105:778–786
Mallett CL, McFadden C, Chen Y, Foster PJ (2012) Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy 14:743–751
de Chickera S, Willert C, Mallet C, Foley R, Foster P et al (2012) Cellular MRI as a suitable, sensitive non-invasive modality for correlating in vivo migratory efficiencies of different dendritic cell populations with subsequent immunological outcomes. Int Immunol 24:29–41
Zhang X, de Chickera SN, Willert C, Economopoulos V, Noad J et al (2011) Cellular magnetic resonance imaging of monocyte-derived dendritic cell migration from healthy donors and cancer patients as assessed in a scid mouse model. Cytotherapy 13:1234–1248
Gonzalez-Lara LE, Xu X, Hofstetrova K, Pniak A, Chen Y et al (2010) The use of cellular magnetic resonance imaging to track the fate of iron-labeled multipotent stromal cells after direct transplantation in a mouse model of spinal cord injury. Mol Imaging Biol
Jirak D, Kriz J, Strzelecki M, Yang J, Hasilo C et al (2009) Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA 22:257–265
Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF et al (2006) In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med 55:23–29
Oweida AJ, Dunn EA, Karlik SJ, Dekaban GA, Foster PJ (2007) Iron-oxide labeling of hematogenous macrophages in a model of experimental autoimmune encephalomyelitis and the contribution to signal loss in fast imaging employing steady state acquisition (FIESTA) images. J Magn Reson Imaging 26:144–151
Bernas LM, Foster PJ, Rutt BK (2010) Imaging iron-loaded mouse glioma tumors with bSSFP at 3 T. Magn Reson Med 64:23–31
Foster PJ, Dunn EA, Karl KE, Snir JA, Nycz CM et al (2008) Cellular magnetic resonance imaging: in vivo imaging of melanoma cells in lymph nodes of mice. Neoplasia 10:207–216
Perera M, Ribot EJ, Percy DB, McFadden C, Simedrea C et al (2012) In vivo magnetic resonance imaging for investigating the development and distribution of experimental brain metastases due to breast cancer. Trans Oncol 5:217–225
Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56:1001–1010
Ribot EJ, Foster PJ (2012) In vivo MRI discrimination between live and lysed iron-labeled cells using balanced steady state free precession. Eur Radiol (in press)
Garden OA, Reynolds PR, Yates J, Larkman DJ, Marelli-Berg FM et al (2006) A rapid method for labelling CD4+ T cells with ultrasmall paramagnetic iron oxide nanoparticles for magnetic resonance imaging that preserves proliferative, regulatory and migratory behaviour in vitro. J Immunol Methods 314:123–133
Beer AJ, Holzapfel K, Neudorfer J, Piontek G, Settles M et al (2008) Visualization of antigen-specific human cytotoxic T lymphocytes labeled with superparamagnetic iron-oxide particles. Eur Radiol 18:1087–1095
Iida H, Takayanagi K, Nakanishi T, Kume A, Muramatsu K et al (2008) Preparation of human immune effector T cells containing iron-oxide nanoparticles. Biotechnol Bioeng 101:1123–1128
Janic B, Rad AM, Jordan EK, Iskander AS, Ali MM et al (2009) Optimization and validation of FePro cell labeling method. PLoS ONE 4:e5873
Arbab AS, Janic B, Jafari-Khouzani K, Iskander AS, Kumar S et al (2010) Differentiation of glioma and radiation injury in rats using in vitro produce magnetically labeled cytotoxic T-cells and MRI. PLoS ONE 5:e9365
Lewin M, Carlesso N, Tung CH, Tang XW, Cory D et al (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18:410–414
Liu L, Ye Q, Wu Y, Hsieh WY, Chen CL et al (2012) Tracking T-cells in vivo with a new nano-sized MRI contrast agent. Nanomedicine [Epub ahead of print]
Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A (2001) Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol 8:1131–1135
Mallett CL, Foster PJ (2011) Optimization of the balanced steady state free precession (bSSFP) pulse sequence for magnetic resonance imaging of the mouse prostate at 3T. PLoS ONE 6:e18361
Lamb LS Jr, Musk P, Ye Z, van Rhee F, Geier SS et al (2001) Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant 27:601–606
Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H et al (2005) Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 201:1567–1578
Schilbach K, Frommer K, Meier S, Handgretinger R, Eyrich M (2008) Immune response of human propagated gammadelta-T-cells to neuroblastoma recommend the Vdelta1+ subset for gammadelta-T-cell-based immunotherapy. J Immunother 31:896–905
Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O et al (2012) Antibody-dependent anti-cytomegalovirus activity of human gammadelta T cells expressing CD16 (FcgammaRIIIa). Blood 119:1418–1427
Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P et al (2010) The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 116:2164–2172
Dokouhaki P, Han M, Joe B, Li M, Johnston MR et al (2010) Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: a new approach. Cancer Lett 297:126–136
Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D et al (2011) Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 118:992–1001
Poggi A, Zocchi MR, Carosio R, Ferrero E, Angelini DF et al (2002) Transendothelial migratory pathways of V delta 1+ TCR gamma delta+ and V delta 2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients: involvement of phosphatidylinositol 3 kinase and calcium calmodulin-dependent kinase II. J Immunol 168:6071–6077
Janssen O, Wesselborg S, Heckl-Ostreicher B, Pechhold K, Bender A et al (1991) T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta+ T cells. J Immunol 146:35–39
Lopez RD, Xu S, Guo B, Negrin RS, Waller EK (2000) CD2-mediated IL-12-dependent signals render human gamma delta-T cells resistant to mitogen-induced apoptosis, permitting the large-scale ex vivo expansion of functionally distinct lymphocytes: implications for the development of adoptive immunotherapy strategies. Blood 96:3827–3837
Viey E, Laplace C, Escudier B (2005) Peripheral gammadelta T-lymphocytes as an innovative tool in immunotherapy for metastatic renal cell carcinoma. Expert Rev Anticancer Ther 5:973–986
Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K et al (2008) Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 10:842–856
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F et al (2003) Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102:200–206
Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C et al (2003) The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat Immunol 4:991–998
Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G et al (2005) Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol 35:1764–1772
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH et al (1999) Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA 96:6879–6884
Novotna B, Jendelova P, Kapcalova M, Rossner P Jr, Turnovcova K et al (2012) Oxidative damage to biological macromolecules in human bone marrow mesenchymal stromal cells labeled with various types of iron oxide nanoparticles. Toxicol Lett 210:53–63
Brekelmans P, van Soest P, Voerman J, Platenburg PP, Leenen PJ et al (1994) Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol 159:331–339
Keating A, Bernstein ID, Papayannopoulou T, Raskind W, Singer JW (1983) EM-2 and EM-3: two new Ph’+ myeloid cell lines. In: PA GDM (ed) Symposia on molecular and cellular biology, new series; UCLA. Alan R. Liss, New York, pp 513–520
Acknowledgments
We would like to extend a heartfelt thank you to our healthy donors, without whom this work would not have been possible. Thanks to Catherine McFadden for advice on cell labeling as well as Gelareh Zadeh and Kelly Burrell for the U87 glioblastoma cells. Additionally, we thank Martin Rutter at Miltenyi Biotec for timely assistance and the Haeryfar laboratory at Western University for lending us the MACS Midi magnet for our depletions. We thank Judith Sholdice for EM imaging. A.K. holds the Gloria and Seymour Epstein Chair in Cell Therapy and Transplantation at University Health Network and the University of Toronto. P.F. was funded by the Ontario Institute for Cancer Research, One Millimeter Cancer Challenge Program.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siegers, G.M., Ribot, E.J., Keating, A. et al. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol Immunother 62, 571–583 (2013). https://doi.org/10.1007/s00262-012-1353-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1353-y