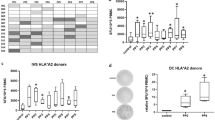
Abstract
Cytotoxic T lymphocytes (CTL) can kill Hodgkin’s lymphoma (HL) cells, and CTL have been used for the treatment of Epstein-Barr virus (EBV)-positive HL. For patients with EBV-negative HL, this strategy cannot be employed and alternative target structures have to be defined. In order to establish a system for the stimulation of HL-reactive T cells, we used dendritic cells (DC) as antigen-presenting cells for autologous T cells and transfected these DC with RNA from established HL cell lines. After stimulation of peripheral blood mononuclear cells (PBMC) with RNA-transfected DC, we analyzed the reactivity of primed PBMC by interferon gamma enzyme-linked immunospot. Our results suggest the presence of antigens with expression in HL cell lines and recognition of these antigens in combination with DC-derived human leukocyte antigen molecules. By the analysis of Gene Expression Omnibus microarray data sets from HL cell lines and primary HL samples in comparison with testis and other normal tissues, we identified HL-associated cancer testis antigens (CTA) including the preferentially expressed antigen in melanoma (PRAME). After stimulation of PBMC with RNA-transfected DC, we detected PRAME-reactive T cells. PRAME and other HL-associated CTA might be targets for HL-specific immune therapy or for the monitoring of HL-directed immune responses.





Similar content being viewed by others
References
Drexler HG, Minowada J (1992) Hodgkin’s disease derived cell lines: a review. Hum Cell 5:42–53
Sahin U, Neumann F, Tureci O, Schmits R, Perez F, Pfreundschuh M (2002) Hodgkin and Reed-Sternberg cell-associated autoantigen CLIP-170/restin is a marker for dendritic cells and is involved in the trafficking of macropinosomes to the cytoskeleton, supporting a function-based concept of Hodgkin and Reed-Sternberg cells. Blood 100:4139–4145
Schwering I, Bräuninger A, Klein U, Jungnickel B, Tinguely M, Diehl V et al (2003) Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 101:1505–1512
Körholz D, Claviez A, Hasenclever D, Kluge R, Hirsch W, Kamprad F et al (2004) The concept of the GPOH-HD 2003 therapy study for pediatric Hodgkin’s disease: evolution in the tradition of the DAL/GPOH studies. Klin Padiatr 216:150–156
Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D et al (2005) Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet 365:1934–1941
Kennedy-Nasser AA, Bollard CM, Rooney CM (2006) Adoptive immunotherapy for Hodgkin’s lymphoma. Int J Hematol 83:385–390
Grunebach F, Muller MR, Brossart P (2005) New developments in dendritic cell-based vaccinations: RNA translated into clinics. Cancer Immunol Immunother 54:517–525
Staege MS, Hansen G, Baersch G, Burdach S (2004) Functional and molecular characterization of interleukin-2 transgenic Ewing tumor cells for in vivo immunotherapy. Pediatr Blood Cancer 43:23–34
Buchner A, Pohla H, Willimsky G, Frankenberger B, Frank R, Baur-Melnyk A et al (2010) Phase 1 trial of allogeneic gene-modified tumor cell vaccine RCC-26/CD80/IL-2 in patients with metastatic renal cell carcinoma. Hum Gene Ther 21:285–297
Mu LJ, Kyte JA, Kvalheim G, Aamdal S, Dueland S, Hauser M et al (2005) Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer 93:749–756
Kyte JA, Kvalheim G, Lislerud K, thor Straten P, Dueland S, Aamdal S et al (2007) T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother 56:659–675
Staege MS, Banning-Eichenseer U, Weissflog G, Volkmer I, Burdach S, Richter G et al (2008) Gene expression profiles of Hodgkin’s lymphoma cell lines with different sensitivity to cytotoxic drugs. Exp Hematol 36:886–896
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33:2643–2652
McAllister RM, Isaacs H, Rongey R, Peer M, Au W, Soukup SW et al (1977) Establishment of a human medulloblastoma cell line. Int J Cancer 20:206–212
Wolf J, Kapp U, Bohlen H, Kornacker M, Schoch C, Stahl B et al (1996) Peripheral blood mononuclear cells of a patient with advanced Hodgkin’s lymphoma give rise to permanently growing Hodgkin-Reed Sternberg cells. Blood 87:3418–3428
Diehl V, Kirchner HH, Schaadt M, Fonatsch C, Stein H, Gerdes J et al (1981) Hodgkin’s disease: establishment and characterization of four in vitro cell lines. J Cancer Res Clin Oncol 101:111–124
Drexler HG, Gaedicke G, Lok MS, Diehl V, Minowada J (1986) Hodgkin’s disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res 10:487–500
Kamesaki H, Fukuhara S, Tatsumi E, Uchino H, Yamabe H, Miwa H et al (1986) Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin’s disease. Blood 68:285–292
Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H et al (1973) In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 51:1417–1423
Salcedo M, Momburg F, Hämmerling GJ, Ljunggren HG (1994) Resistance to natural killer cell lysis conferred by TAP1/2 genes in human antigen-processing mutant cells. J Immunol 152:1702–1708
Foell JL, Volkmer I, Giersberg C, Kornhuber M, Horneff G, Staege MS (2008) Loss of detectability of Charcot-Leyden crystal protein transcripts in blood cells after treatment with dimethyl sulfoxide. J Immunol Methods 339:99–103
Meyer-Wentrup F, Burdach S (2003) Efficacy of dendritic cell generation for clinical use: recovery and purity of monocytes and mature dendritic cells after immunomagnetic sorting or adherence selection of CD14+ starting populations. J Hematother Stem Cell Res 12:289–299
Foell JL, Hesse M, Volkmer I, Schmiedel BJ, Neumann I, Staege MS (2008) Membrane-associated phospholipase A1 beta (LIPI) is an Ewing tumour-associated cancer/testis antigen. Pediatr Blood Cancer 51:228–234
Neumann I, Foell JL, Bremer M, Volkmer I, Korholz D, Burdach S et al (2010) Retinoic acid enhances sensitivity of neuroblastoma cells for imatinib mesylate. Pediatr Blood Cancer 55:464–470
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM et al (2005) Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 86:127–141
Barrett T, Troup DB, Wilhite SE, Ledoux P, Evangelista C, Kim IF et al. (2011) NCBI GEO: archive for functional genomics data sets–10 years on. Nucleic Acids Res 39 Database issue:D1005-1010
Sturn A, Quackenbush J, Trajanoski Z (2002) Genesis: cluster analysis of microarray data. Bioinformatics 18:207–208
Dangond F, Hwang D, Camelo S, Pasinelli P, Frosch MP, Stephanopoulos G et al (2004) Molecular signature of late-stage human ALS revealed by expression profiling of postmortem spinal cord gray matter. Physiol Genomics 16:229–239
Brune V, Tiacci E, Pfeil I, Döring C, Eckerle S, van Noesel CJ et al (2008) Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J Exp Med 205:2251–2268
Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC et al (2006) Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 7:67–80
Eckerle S, Brune V, Döring C, Tiacci E, Bohle V, Sundström C et al (2009) Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia 23:2129–2138
Köchert K, Ullrich K, Kreher S, Aster JC, Kitagawa M, Jöhrens K et al (2010) High-level expression of Mastermind-like 2 contributes to aberrant activation of the NOTCH signaling pathway in human lymphomas. Oncogene 30:1831–1840
Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P et al (2011) MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 471:377–381
Liu TY, Wu SJ, Huang MH, Lo FY, Tsai MH, Tsai CH et al (2010) EBV-positive Hodgkin lymphoma is associated with suppression of p21cip1/waf1 and a worse prognosis. Mol Cancer 9:32
Hoennscheidt C, Max D, Richter N, Staege MS (2009) Expression of CD4 on Epstein-Barr virus-immortalized B cells. Scand J Immunol 70:216–225
Ghafouri-Fard S, Abbasi A, Moslehi H, Faramarzi N, Taba Taba Vakili S, Mobasheri MB et al (2010) Elevated expression levels of testis-specific genes TEX101 and SPATA19 in basal cell carcinoma and their correlation with clinical and pathological features. Br J Dermatol 162:772–779
Chen YT, Chadburn A, Lee P, Hsu M, Ritter E, Chiu A et al (2010) Expression of cancer testis antigen CT45 in classical Hodgkin lymphoma and other B-cell lymphomas. Proc Natl Acad Sci USA 107:3093–3098
Willenbrock K, Küppers R, Renné C, Brune V, Eckerle S, Weidmann E et al (2006) Common features and differences in the transcriptome of large cell anaplastic lymphoma and classical Hodgkin’s lymphoma. Haematologica 91:596–604
Thorisson GA, Smith AV, Krishnan L, Stein LD (2005) The international HapMap project Web site. Genome Res 15:1592–1593
Ikeda H, Lethé B, Lehmann F, van Baren N, Baurain JF, de Smet C et al (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6:199–208
Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM et al (2001) Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med 193:73–88
Quintarelli C, Dotti G, Hasan ST, De Angelis B, Hoyos V, Errichiello S et al (2011) High-avidity cytotoxic-T-lymphocytes specific for a new preferentially expressed antigen of melanoma (PRAME)-derived peptide can target leukemic- and leukemic-precursor cells. Blood 117:3353–3362
Kawahara M, Hori T, Matsubara Y, Okawa K, Uchiyama T (2006) Identification of HLA class I-restricted tumor-associated antigens in adult T cell leukemia cells by mass spectrometric analysis. Exp Hematol 34:1496–1504
Parmiani G, De Filippo A, Novellino L, Castelli C (2007) Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol 178:1975–1979
Gao L, Downs AM, Stauss HJ (2005) Immunotherapy with CTL restricted by nonself MHC. Methods Mol Med 109:215–228
Lin W, Lai CH, Tang CJ, Huang CJ, Tang TK (1999) Identification and gene structure of a novel human PLZF-related transcription factor gene, TZFP. Biochem Biophys Res Commun 264:789–795
Kaufmann S, Sauter M, Schmitt M, Baumert B, Best B, Boese A et al (2010) Human endogenous retrovirus protein Rec interacts with the testicular zinc-finger protein and androgen receptor. J Gen Virol 91:1494–1502
Lamprecht B, Walter K, Kreher S, Kumar R, Hummel M, Lenze D et al (2010) Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med 16:571–579
Khong HT, Wang QJ, Rosenberg SA (2004) Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother 27:184–190
Oehler VG, Guthrie KA, Cummings CL, Sabo K, Wood BL, Gooley T et al (2009) The preferentially expressed antigen in melanoma (PRAME) inhibits myeloid differentiation in normal hematopoietic and leukemic progenitor cells. Blood 114:3299–3308
Ortmann CA, Eisele L, Nückel H, Klein-Hitpass L, Führer A, Dührsen U et al (2008) Aberrant hypomethylation of the cancer-testis antigen PRAME correlates with PRAME expression in acute myeloid leukemia. Ann Hematol 87:809–818
Morandi F, Chiesa S, Bocca P, Millo E, Salis A, Solari M et al (2006) Tumor mRNA-transfected dendritic cells stimulate the generation of CTL that recognize neuroblastoma-associated antigens and kill tumor cells: immunotherapeutic implications. Neoplasia 8:833–842
Acknowledgments
This work was supported by grants from Peter-Escher-Foundation for children with cancer.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Winkler, C., Steingrube, D.S., Altermann, W. et al. Hodgkin’s lymphoma RNA-transfected dendritic cells induce cancer/testis antigen-specific immune responses. Cancer Immunol Immunother 61, 1769–1779 (2012). https://doi.org/10.1007/s00262-012-1239-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1239-z