Abstract
Attenuated and heat-killed mycobacteria display demonstrable activity against cancer in the clinic; however, the induced immune response is poorly characterised and potential biomarkers of response ill-defined. We investigated whether three mycobacterial preparations currently used in the clinic (BCG and heat-killed Mycobacterium vaccae and Mycobacterium obuense) can stimulate anti-tumour effector responses in human γδ T-cells. γδ T-cell responses were characterised by measuring cytokine production, expression of granzyme B and cytotoxicity against tumour target cells. Results show that γδ T-cells are activated by these mycobacterial preparations, as indicated by upregulation of activation marker expression and proliferation. Activated γδ T-cells display enhanced effector responses, as shown by upregulated granzyme B expression, production of the TH1 cytokines IFN-γ and TNF-α, and enhanced degranulation in response to susceptible and zoledronic acid-treated resistant tumour cells. Moreover, γδ T-cell activation is induced by IL-12, IL-1β and TNF-α from circulating type 1 myeloid dendritic cells (DCs), but not from type 2 myeloid DCs or plasmacytoid DCs. Taken together, we show that BCG, M. vaccae and M. obuense induce γδ T-cell anti-tumour effector responses indirectly via a specific subset of circulating DCs and suggest a mechanism for the potential immunotherapeutic effects of BCG, M. vaccae and M. obuense in cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune system’s intrinsic ability to recognise and destroy tumour cells is an attractive target for therapeutic intervention. Moreover, cancer patients are often immunocompromised due to the immunosuppressive nature of the tumour and collateral affects of certain chemotherapies. Cancer immunotherapy aims to bolster appropriate anti-tumour immune responses; an approach that has great potential as a stand-alone treatment or combinatorial partner of standard chemotherapy. Indeed, cancer immunotherapy has had many successes, with one of the most prominent milestones to date being the use of Bacillus Calmette Guérin (BCG) to treat bladder cancer [1].
The remarkable success of BCG therapy in bladder cancer highlights the potential for mycobacterial preparations as cancer immunotherapeutic agents or as adjuvants to existing chemotherapies. There has also been considerable interest in a heat-killed preparation of Mycobacterium vaccae for the treatment of cancers of the prostate, lung, kidney and skin [2–5]. In particular, M. vaccae combined with chemotherapy increased survival and improved quality of life in phase III trials for adenocarcinoma of the lung [6, 7]. Recently, attention has turned to heat-killed preparations of M. obuense, which are currently undergoing early phase trials in melanoma [8]. Although mycobacterial preparations are currently under clinical investigation, mechanistic studies are limited and biomarkers of response ill-defined, which is hampering their success. In vitro studies are needed to identify candidate immune cells for immunological monitoring during clinical trials. γδ T-cells have a number of properties that highlight them as a potential mechanism by which mycobacterial preparations elicit their anti-cancer effects. In particular, γδ T-cells play a demonstrable role in protective immunosurveillance against cancer [9–11]; are highly reactive against mycobacterial antigens [12]; and display potent cross-reactivity against tumour cells and mycobacteria [13]. However, the capacity for clinical preparations of BCG, M. vaccae and M. obuense to elicit effector responses in γδ T-cells has been largely overlooked and remains poorly characterised.
γδ T-cells can elicit protective immune responses against cancer and are an essential component of the anti-tumour immune response. In vitro, human γδ T-cells display potent cytotoxicity against tumour cells from a broad range of epithelial and haematalogic malignancies [14–16]. They also produce interferon (IFN)-γ and tumour necrosis factor (TNF)-α in response to mycobacteria and tumour, which potentiate protective cell-mediated immune responses against cancer [17]. Moreover, γδ T-cell responses to antigenic challenge are rapid and memory-like, thus providing an early defence mechanism that complements the delayed immune response of αβ T-cells [18]. In contrast to αβ T-cells, γδ T-cells recognise phosphoantigens independently of major histocompatibility complex (MHC) class I, which is often down-modulated in a range of cancers, thus reinforcing the value of γδ T-cells in cancer immunotherapy [19]. γδ T-cells also express the natural killer activatory receptor NKG2D; this receptor interacts with MHC class I-related stress molecules such as MICA and MICB, which are frequently upregulated on tumours [20].
Our aim was to examine whether BCG and heat-killed M. vaccae and M. obuense can prime γδ T-cells for an anti-tumour effect. Data presented herein suggest that these mycobacterial preparations stimulate anti-tumour responses in γδ T-cells, as shown by production of TH1 cytokines, upregulation of granzyme B and increased cytotoxicity against tumour cells. Furthermore, data suggest that γδ T-cell responses are indirectly stimulated by IL-12, IL-1β and TNF-α from circulating type 1 myeloid dendritic cells (mDC1s). Taken together, our study is the first to demonstrate that BCG, M. vaccae and M. obuense may enhance the effector responses of γδ T-cells by stimulating mDC1s to produce IL-12, IL-1β and TNF-α, which sheds light on the mechanism of action for the anti-cancer effects of these immunotherapies.
Materials and methods
Mycobacteria
Heat-killed M. vaccae and M. obuense were supplied by Professor John Stanford (University College London). Mycobacteria were heat-killed by autoclaving at 121°C for 15 min in borate-buffered solution. Lyophilised BCG vaccine (Danish strain 1331; Statens Serum Institut) was resuspended in phosphate-buffered saline (PBS; Sigma) and heat-killed as described above. Mycobacteria were added to cell cultures using optimised doses of 1 × 105 culturable particles/ml BCG, 100 μg/ml M. vaccae and 100 μg/ml M. obuense (supplementary fig. 1).
Cell isolation/depletion
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors by density-adjusted centrifugation using Histopaque −1,077 (Sigma). Contaminating red blood cells were lysed with hypotonic ammonium chloride (5 Prime) and platelets removed by centrifugation at 200 g. Specific cell populations were isolated or depleted from PBMCs using magnetic microbeads against TCRγδ, CD14, CD4, CD8, CD19, CD56 and/or CD1c (Miltenyi Biotec) according to the manufacturer’s instructions. Purities for cell isolations were analysed by flow cytometry and were consistently >95%.
Cell culture
All cell cultures were performed at 37°C with 5% CO2. 1 × 106 PBMCs in 200 μl complete medium RPMI-1640 (Sigma) with 5% heat-inactivated human A/B serum (Lonza) and 2 mM l-glutamine (Sigma), were cultured in 96-well, flat-bottomed tissue culture plates. For proliferation assays, PBMCs were stained with 400 nM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and 1 × 106 cells in 1 ml of complete medium cultured in 24-well tissue culture plates for 6 days. The percentage of cells with CFSE fluorescence lower than the untreated controls was used as a measure of proliferation. 2–5 × 104 purified γδ T-cells in 200 μl of complete medium were cultured in 96-well, round-bottomed tissue culture plates alone or with CD56+, CD4+, CD8+ or CD1c+ cells. For some experiments, γδ T-cells were cultured overnight with recombinant human IL-12 (Miltenyi Biotec), IL-1β and TNF-α (both Peprotech) at concentrations indicated in figure legends. 1 × 106 CD4+ cells in 200 μl complete medium were cultured in 96-well, flat-bottomed tissue culture plates. For some experiments, supernatants were collected and passed through 0.2-μm filters prior to use. For intracellular cytokine staining, 1 μg/ml of Brefeldin A (Sigma) was added for the last 4 h of culture.
The following positive controls were used: isopentenyl pyrophosphate (IPP; 10 μg/ml); phorbol myristate acetate (PMA; 25 ng/ml); ionomycin (I; 1 μg/ml); phytohaemagglutinin-leucoagglutinin (PHA-L; 1 μg/ml); lipopolysaccharide (LPS; E. coli-derived; 1 μg/ml); R848 (2.5 μg/ml) (all from Sigma); and IL-2 (5U/ml; Peprotech).
The Burkitt’s Lymphoma cell line Daudi and lung carcinoma cell line A549 (both ECACC) were cultured in RPMI-1640 or Dulbecco’s modified eagle media (DMEM; Sigma), respectively, supplemented with 10% foetal bovine serum (FBS; Invitrogen) and 2 mM l-glutamine. 2 × 106 A549 cells were added per 75 cm2 tissue culture flask and allowed to adhere for 24 h prior to overnight culture with 0.1, 1 or 10 μM of zoledronic acid (Novartis).
Flow cytometry
Cells were stained in buffer (PBS with 1% albumin bovine serum (Sigma) and 0.1% sodium azide (Sigma) for 30 min at 4°C with the following monoclonal antibodies: CD3-FITC, CD3-PERCP, TCRγδ-PE, Vδ2-PE, CD69-FITC, CD69-PE, CD69-APC, HLA-DR-PERCP, CD25-APC, CD4-PE, CD123-PE, CD123-PECy5 and CD14-APC (all from Becton–Dickinson); CD11c-FITC, CD11c-PE and CD1c-PE (all from Miltenyi Biotec); and Vδ1-FITC (Thermo Scientific). Matched isotype control antibodies were used to determine background staining. Cells were then fixed in 4% paraformaldehyde (BD Cellfix; Becton–Dickinson) for 20 min at 4°C. For intracellular cytokine staining, cells were simultaneously fixed and permeabilised in 4% paraformaldehyde and 0.1% saponin (BD Cytofix/Cytoperm; Becton–Dickinson) for 20 min at 4°C and then resuspended in 0.1% saponin with IFN-γ-APC, TNF-α-APC, IL-10-APC, IL-12-APC (all from Miltenyi Biotec), IFN-γ-PECy7 (Becton–Dickinson), IL-1β-APC (R and D Systems) or Granzyme B-APC (Caltag-Medsystems) for 10 min at room temperature. Expression levels were analysed using FACsCalibur or LSRII with CellQuest Pro or FACs DIVA software, respectively (all Becton–Dickinson).
Tritiated 3H incorporation
Tritiated [3H]-thymidine incorporation was used to assess proliferation of purified γδ T-cells. Cells were cultured with mycobacteria for 6 days prior to the addition of 2.5 μCi/ml [3H]-thymidine (GE Healthcare) for the last 18 h of culture. Cells were osmotically lysed and DNA collected onto filters (Perkin Elmer). Scintillation fluid (Perkin Elmer) was added prior to measuring beta emission (photon counts per minute) using a liquid scintillation counter (Perkin Elmer).
Cytokine analysis of culture supernatants
TH1/TH2 cytometric bead arrays (CBAs; Becton–Dickinson) were used to measure the concentration of IFN-γ, TNF-α, IL-10, IL-5, IL-4 and IL-2 in cell culture supernatants according to the manufacturer’s instructions. Supernatants were also analysed by multi-analyte profiling of inflammation-related analytes by Rules Based Medicine (USA).
Cytotoxicity assays
Mycobacteria-stimulated PBMCs were co-cultured with Daudi or zoledronic acid-treated A549 cells at a pre-optimised effector:target cell ratio of 2:1. Antibodies to CD107b-FITC and CD107a-APC or IgG1κ-FITC and IgG1κ-APC (Becton–Dickinson) were added directly to the wells. Co-cultures were incubated for 1 h prior to addition of 1 μg/ml monensin (Sigma) to neutralise intracellular acidity. After a further 5 h of culture, Vδ2 T-cell expression of CD107a and CD107b was assessed by flow cytometry. Alternatively, cytokine-treated γδ T-cells were co-cultured overnight with Daudi cells at target:effector cell ratios of 1:10, 1:20 and 1:50 in 96-well, V-bottomed tissue culture plates. Cytotoxicity was assessed using the lactate dehydrogenase assay (Promega) according to the manufacturer’s instructions. Percentage cytotoxicity was calculated against maximum target cell release using 9% Triton X-100 (Promega).
Blocking experiments
PBMCs were cultured overnight with mycobacteria in the presence of blocking antibodies to IL-12, IL-1β and TNF-α (R and D Systems) each at 100 μg/ml. Also, culture supernatants from mycobacteria-treated CD4+ cells were pre-treated for 1 h with blocking antibodies to IL-12, IL-1β and/or TNF-α each at 100 μg/ml prior to culturing with γδ T-cells. Goat IgG1 isotype antibodies (R and D Systems) were used as controls throughout.
Statistical analyses
Statistical testing was carried out using SigmaStat (SPSS Inc.) analytical software. Statistical differences between conditions were determined using one-way ANOVA (for parametric data with equal variances and normal distributions) or Kruskal–Wallis ANOVA on ranks (for non-parametric data) followed by either Holm-Sidak or Dunn’s Tests, respectively. For some experiments, statistical differences between conditions were determined using the student’s paired t test. Differences with P values less than 0.05 were deemed significant.
Results
γδ T-cells within mycobacteria-treated PBMCs produce granzyme B and TH1 cytokines
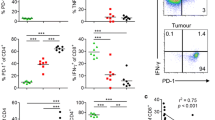
To determine the effects of mycobacterial adjuvants on γδ T-cells, we measured activation, proliferation, granzyme B expression and cytokine production by γδ T-cells within BCG-, M. vaccae- and M. obuense-treated PBMCs. Preliminary experiments show that live and heat-killed BCG were equipotent at upregulating CD69 on γδ T-cells (supplementary fig. 1a). Using the pre-optimised concentrations determined in supplementary figs. 1a and 1b, heat-killed BCG, M. vaccae and M. obuense upregulated CD69, CD25 and HLA-DR expression on γδ T-cells (Fig. 1a). Activation was restricted to the Vδ2+ subset, as shown by upregulated CD69 expression in Vδ2+ but not Vδ1+ cells (Fig. 1b). The mycobacteria induced proliferation in γδ T-cells; however, levels of proliferation against BCG and M. obuense were markedly higher than against M. vaccae (Fig. 1c). Changes in the percentage of γδ T-cells within PBMCs after 6 days of mycobacterial stimulation were only minor and not significant (data not shown), suggesting that other cell types are also proliferating. Moreover, the mycobacteria upregulated γδ T-cell expression of granzyme B (Fig. 1d). Analysis of supernatants from mycobacteria-treated PBMCs indicated detectable levels of IFN-γ, TNF-α and IL-10, but relatively low levels of IL-5, IL-4 and IL-2 (data not shown). Intracellular cytokine staining revealed that γδ T-cells produced IFN-γ and TNF-α, but not IL-10 (Fig. 1e).
γδ T-cells within BCG-, M. vaccae- and M. obuense-treated PBMCs produce granzyme B and TH1 cytokines. PBMCs were cultured with heat-killed BCG, M. vaccae (Mv) and M. obuense (Mo) and responses measured within gated γδ T-cells. Untreated (un) cells were used as a negative control. a Gating strategy used to identify γδ T-cells within PBMCs. Lymphocytes were gated according to size (forward scatter; FSC) and granularity (side scatter; SSC). Within lymphocytes, γδ T-cells were gated as CD3+TCRγδ+. Mean percentages of γδ T-cells expressing CD69 (at 24 h; n = 5), CD25 and HLA-DR (both at 48 h; n = 3) are shown. b Mean percentages of Vδ1+ and Vδ2+ cells expressing CD69 (at 24 h; n = 3). c CFSE+ PBMCs were cultured for 6 days with mycobacteria and proliferation measured within the γδ T-cell compartment. Mean percentages of γδ T-cells proliferating are shown (n = 6). d Mean fluorescent intensities (MFI) of granzyme B expression within γδ T-cells (at 24 h; n = 3). e Percentages of γδ T-cells expressing IFN-γ, TNF-α and IL-10 were measured after 24 h of stimulation. Representative flow cytometric dot plots from one donor and mean values for n = 3 are shown. Error bars represent SD. * and ** indicates P values of <0.05 and <0.001, respectively, for statistical comparisons between treated and untreated cells
Mycobacteria-activated Vδ2+ cells have enhanced cytotoxicity against tumour cells
Upregulation of granzyme B suggests that mycobacteria-activated γδ T-cells may have enhanced cytotoxic properties. We investigated the ability of mycobacteria-activated γδ T-cells to degranulate in the presence of tumour cells. The Burkitt’s lymphoma cell line Daudi and the lung cancer cell line A549 were selected as γδ T-cell susceptible and resistant tumour target cells, respectively. Resting Vδ2+ cells increased CD107a/b expression when exposed to Daudi but not A549 target cells (Fig. 2a). Mycobacteria-activated Vδ2+ cells had higher levels of Daudi-induced CD107a/b expression than untreated Vδ2+ cells (Fig. 2b–d). Pre-treating A549 cells with zoledronic acid increased their capacity to induce degranulation in resting Vδ2+ cells (Fig. 2e). Moreover, mycobacteria-activated Vδ2+ cells had increased percentage expression of CD107a/b and mean fluorescence intensity (MFI) expression of CD107a in response to A549 cells pre-treated with zoledronic acid (Fig. 2e). Taken together, mycobacterial adjuvants enhance γδ T-cell degranulation in the presence of susceptible tumours but further treatment was required to expose this effect in tumours that are refractory to γδ T-cell killing.
BCG, M. vaccae and M. obuense enhance γδ T-cell cytotoxicity. PBMCs were cultured overnight with heat-killed BCG, M. vaccae (Mv) and M. obuense (Mo). Untreated (un) cells were used as a negative control. PBMCs were then co-cultured for 6 h with Daudi or A549 cells at an effector:target cell ratio of 2:1 and CD107a/b expression measured within gated Vδ2+ cells. a Mean percentage of Vδ2+ cells expressing CD107a/b in the absence (media) or presence of Daudi or A549 target cells (n ≥ 5). b Representative flow cytometric dot plots from one donor showing CD107a/b expression on gated Vδ2+ cells. c Mean percentage of Vδ2+ cells expressing CD107a/b in the absence (Media) or presence of Daudi target cells (n = 5). d Media test scores were subtracted from Daudi test scores. Mean values are shown for n = 5. Error bars represent SD and * indicates a P value of <0.05 for statistical comparisons between untreated and treated conditions. For statistical testing, data were standardised by subtracting the untreated scores from test scores. e Percentage of Vδ2+ cells expressing CD107a/b and mean fluorescent intensity (MFI) expression of CD107a on Vδ2+ cells in the presence of zoledronic acid-treated A549 cells. Background levels of degranulation were subtracted from A549-induced degranulation. Individual experiments for three donors are shown
Vδ2+ cell responses to mycobacteria are dependent on CD4+ cells
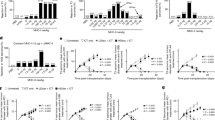
A range of cell types have been implicated in mediating γδ T-cell activation [21–23]. To determine whether the effect of BCG, M. vaccae and M. obuense on γδ T-cells is direct or mediated by another cell type, the capacity for these mycobacterial preparations to activate highly purified γδ T-cells was assessed. Purified γδ T-cells (mean ± SD: 98.2 ± 1.1%) were not activated by the mycobacteria, as shown by baseline levels of CD69 expression, cytokine production and proliferation (Fig. 3a–c). To determine the cell type required for activation, PBMCs were sequentially depleted of CD14+ cells (monocytes), CD19+ cells (B-cells) and CD56+ cells (NK and NKT cells) prior to overnight stimulation and analysis of CD69 expression on Vδ2+ cells. Depletion of CD14+ monocytes augmented mycobacteria-induced Vδ2+ cell expression of CD69 (Fig. 3d) and IFN-γ (data not shown), suggesting that in our system monocytes are inhibitory. Furthermore, co-culturing γδ T-cells with monocytes failed to restore their response to the mycobacteria (data not shown). Subsequent depletion of CD19+ B-cells had no effect, whereas depletion of CD56+ NK and NKT cells reduced mycobacteria-induced CD69 expression on Vδ2+ cells (Fig. 3d). Purified γδ T-cells were then stimulated with mycobacteria in the presence of either CD56+ cells or a combination of CD4+ and CD8+ cells prior to flow cytometric analysis of IFN-γ production. In the presence of CD4+/CD8+ cells but not CD56+ cells, the mycobacteria induced IFN-γ production by Vδ2+ cells (Fig. 3e). Furthermore, CD4+ but not CD8+ cells were responsible for restoring γδ T-cell responses to the mycobacterial preparations (Fig. 3f).
BCG-, M. vaccae- and M. obuense-induced activation of γδ T-cells is dependent on CD4+ cells. a γδ T-cells were cultured overnight with PMA/I, heat-killed BCG, M. vaccae (Mv) and M. obuense (Mo) and CD69 expression measured. Representative flow cytometric histogram plots from one of two donors are shown. Expression for untreated cells is shown in grey fill. b Culture supernatants from γδ T-cells were screened for their cytokine content using cytometric bead arrays. Mean values for n = 3 are shown. c γδ T-cells were cultured with PHA/IL-2, BCG, Mv and Mo for 6 days and 3H incorporation measured for the last 16 h of culture. Mean counts per minute (CPM) are shown for n = 2. d PBMCs were sequentially depleted of CD14+, CD19+ and CD56+ cells and stimulated overnight with BCG, Mv and Mo prior to measuring CD69 expression on gated Vδ2+ cells. Data were standardised by subtracting untreated scores from test scores. Mean values for n = 3 are shown. e γδ T-cells were co-cultured overnight with CD56+ cells (top graph) or a combination of CD4+ and CD8+ cells (bottom graph) in the presence of mycobacteria prior to measuring IFN-γ expression on gated Vδ2+ cells. Mean values for n ≥ 2 are shown. f γδ T-cells were co-cultured overnight with either CD8+ (top graph) or CD4+ (bottom graph) in the presence of mycobacteria prior to measuring IFN-γ expression on gated Vδ2+ cells. Mean values for n = 3 are shown. Error bars represent SD
Vδ2+ cells are activated by IL-12, IL-1β and TNF-α released from mycobacteria-stimulated CD4+ cells
We assessed whether soluble mediators are responsible for γδ T-cell activation. Culture supernatants from mycobacteria-stimulated CD4+ cells were added to purified γδ T-cells, and IFN-γ production by the Vδ2+ subset was measured. Results show that culture supernatants from treated CD4+ cells upregulated IFN-γ production by Vδ2+ cells (Fig. 4a). Multi-analyte profiling of culture supernatants from mycobacteria-treated CD4+ cells revealed the presence of a number of cytokines (data not shown). Of these, IL-12, IL-1 and TNF have been previously shown to mediate γδ T-cell activation [24–26]. To determine the role played by these three cytokines, PBMCs were stimulated with mycobacteria in the presence of blocking antibodies to IL-12, IL-1β and TNF-α. This resulted in a marked reduction in Vδ2+ cell IFN-γ production (Fig. 4b), indicating that these cytokines are key mediators in γδ T-cell activation. To confirm this observation, isolated γδ T-cells were stimulated with conditioned media from mycobacteria-treated CD4+ cells in the presence of blocking antibodies to IL-12, IL-1β and TNF-α. Results show that blocking these cytokines reduced Vδ2+ cell IFN-γ production (Fig. 4c, d). Analysis of these blocking antibodies either individually or in various combinations revealed a combined effect of all three cytokines (Fig. 4d).
IL-12, IL-1β and TNF-α from BCG-, M. vaccae- and M. obuense-treated CD4+ cells activate Vδ2+ cells. a CD4+ cells were cultured overnight with heat-killed BCG, M. vaccae (Mv) and M. obuense (Mo). γδ T-cells were then cultured overnight in culture supernatant (spnt) from treated CD4+ cells prior to measuring IFN-γ expression on gated Vδ2+ cells. IFN-γ expression was also measured on γδ T-cells that had been cultured directly with mycobacteria in the presence or absence of CD4+ cells. Data were standardised by subtracting untreated scores from test scores. b PBMCs were cultured overnight with mycobacteria in the presence of blocking antibodies to IL-12, IL-1β and TNF-α each at 100 μg/ml. 300 μg/ml of isotype control antibodies were used as a control. IFN-γ expression on gated Vδ2+ cells was then measured. c γδ T-cells were cultured overnight with spnts from mycobacteria-treated CD4+ cells in the presence of blocking antibodies to IL-12, IL-1β and TNF-α each at 100 μg/ml prior to measuring IFN-γ expression on gated Vδ2+ cells. Representative flow cytometric dot plots are shown. d Mean percentage of Vδ2+ cells expressing IFN-γ relative to isotype control for experiments conducted in c. Data points are mean values (n = 7 for a; n = 3 for Mv and Mo in b; n = 2 for BCG in b; and n = 3 for d) where error bars represent SD and * and ** indicate P values of <0.05 and <0.001, respectively, for statistical comparisons between treated and untreated cells
To confirm the key role of these cytokines, we measured γδ T-cell IFN-γ production, granzyme B expression and cytotoxicity in response to recombinant IL-12, IL-1β and TNF-α. γδ T-cells cultured with all three cytokines resulted in the greatest IFN-γ response in the Vδ2+ subset (Fig. 5a). In accordance with data shown in Fig. 1b, these cytokines did not induce IFN-γ production in Vδ2− γδ T-cells (Fig. 5a). IL-12, IL-1β and TNF-α upregulated intracellular expression of granzyme B (Fig. 5b), yet did not induce degranulation, as shown by baseline levels of CD107a expression (data not shown). γδ T-cells activated with these cytokines showed a marked increase in cytotoxicity against Daudi target cells (Fig. 5c). Together, these data demonstrate that IL-12, IL-1β and TNF-α can induce effector responses in γδ T-cells.
IL-12, IL-1β and TNF-α induce γδ T-cell IFN-γ production and cytotoxicity. a γδ T-cells were cultured overnight with different concentrations and combinations of IL-12, IL-1β and TNF-α prior to measuring IFN-γ expression on gated Vδ2+ and Vδ2− cells. Individual experiments from three donors are shown. b MFIs for granzyme B expression on gated γδ T-cells after overnight culture with 10 ng/ml each of IL-12, IL-1β and TNF-α. Left: Representative flow cytometric histogram plots from one donor. Black fill is the isotype control antibody. Numbers are MFIs within the marker shown. Right: MFI values for n = 3. c Cytokine-activated γδ T-cells were co-cultured overnight with Daudi cells at the target (T) to effector (E) cell ratios shown. Cytotoxicity was assessed by measuring lactate dehydrogenase release into the culture supernatants. Mean values for n = 3 (T:E 1:50) and n = 2 (T:E 1:10 and 1:20) are shown. Untreated (un) cells were used as a negative control throughout. Error bars represent SD deviations and * indicates a P value of <0.05 for paired t tests between treatments at the T:E ratio of 1:50
IL-12, IL-1β and TNF-α are produced by CD4+ type 1 myeloid DCs
We have determined the importance of CD4+ cells and their cytokines in mycobacteria-induced stimulation of γδ T-cells. However, CD4 is predominantly expressed on αβ T-cells, which typically do not produce IL-12 or IL-1β. These cytokines are mostly associated with antigen presenting cells (APCs) such as monocytes and DCs, which express low levels of CD4. Therefore, we assessed whether APCs are present in the CD4+ cell population and measured intracellular expressions of IL-12, IL-1β and TNF-α. Monocytes were depleted from the PBMC preparations prior to CD4+ cell isolation and therefore were not present in the CD4+ cell population. Flow cytometric analysis of CD4, CD3, CD11c, CD123 and CD14 revealed three populations of cells: T-cells (CD4highCD3+), myeloid DCs (mDCs; CD4lowCD3−CD11c+CD123−/lowCD14−) and plasmacytoid DCs (pDCs; CD4lowCD3−CD11c−CD123highCD14−) (Fig. 6a). Intracellular cytokine staining of mycobacteria-treated CD4+ cells revealed that IL-12, IL-1β and TNF-α were expressed predominantly in mDCs (Fig. 6b).
IL-12, IL-1β and TNF-α are produced by type 1 myeloid DCs. a Flow cytometry was used to measure the expression of CD4, CD3, CD11c, CD123 and CD14 on purified CD4+ cells. From left to right: Debris was excluded according to size and granularity using gate (G) 1. Within the G1 population, CD3 and CD4 expression identified CD4+ αβ T-cells (CD3+CD4high) and non-T-cells (CD3−CD4low; G2). Within the G1+G2 non-T-cell population, CD11c and CD123 expression identified myeloid DC (mDC) and plasmacytoid DC (pDC) populations, both of which did not express CD14. Representative flow cytometric dot plots from one of two donors are shown. b CD4+ cells were cultured overnight with LPS/R848, heat-killed BCG, M. vaccae (Mv) and M. obuense (Mo). Untreated (un) cells were used as a negative control. IL-12, IL-1β and TNF-α expression was measured on gated T-cells (CD3+), mDCs (CD3−CD11c+) and pDCs (CD3−CD11c−CD123high). Mean values for n = 3 are shown (except TNF-α, where n = 2). Error bars represent SD. * and ** indicate P values of <0.05 and <0.001, respectively, for statistical comparisons between treated and untreated conditions. c CD1c expression was analysed on gated mDCs as shown. d Top panels: CD1c+ cells were depleted from the CD4+ cell population. Representative data from one of three donors are shown. Bottom panels: γδ T-cells were co-cultured overnight with CD4+ cells or CD1c-depleted CD4+ cells in the absence or presence of BCG. Percentage of Vδ2+ cells expressing IFN-γ was measured
Two types of mDC have been defined based on expression of CD123: type 1 (CD123low; mDC1) and type 2 (CD123− mDC2) [27]. Analysis of CD123 expression revealed that CD123+ but not CD123− mDCs produced IL-12, IL-1β and TNF-α in response to the mycobacteria, suggesting that mDC1s and not mDC2s are involved in the γδ T-cell response to these mycobacterial preparations (data not shown). Accordingly, the majority of the mDC population expressed CD1c, a specific marker for mDC1s (Fig. 6c). To confirm the role of mDC1s in the γδ T-cell response to mycobacteria, we depleted CD1c+ cells from the CD4+ cell population and assessed the effects on γδ T-cell activation. Results show that when CD1c+ cells were depleted from γδ T-cell and CD4+ cell co-cultures, IFN-γ production by Vδ2+ cells in response to mycobacteria was lost (Fig. 6d). Taken together, data suggest that mDC1s are activated by mycobacteria to produce cytokines that activate γδ T-cell responses.
Discussion
As part of our ongoing studies into the mechanisms of action for BCG, M. vaccae and M. obuense cancer immunotherapy, we investigated the potential role of γδ T-cells. Our data suggest that BCG, M. vaccae and M. obuense activate an anti-tumour programme in peripheral blood Vδ2+ γδ T-cells that is characterised by TH1 cytokine production and enhanced cytotoxic responses against tumour. Moreover, our data suggest that these responses are indirectly mediated by IL-12, IL-1β and TNF-α from circulating mDC1s.
γδ T-cells are highly responsive to mycobacteria; however, studies have primarily focussed on the stimulatory capacity of live mycobacterial infections and lysate preparations. We focussed on live attenuated BCG and heat-killed M. vaccae and M. obuense, which are preparations of mycobacteria that are currently used as cancer immunotherapies in the clinic. Little is known about whether these preparations can stimulate anti-tumour effector responses in γδ T-cells nor the mechanisms involved. We show here that BCG-, M. vaccae- and M. obuense-activated PBMCs contain γδ T-cells that produce the TH1 cytokines IFN-γ and TNF-α, both of which play a demonstrable role in anti-tumour immunity. Documented effects of these cytokines include the following: MHC class I upregulation on tumour cells, which enhances recognition by cytotoxic αβ T-cells [28, 29]; cell cycle blockade and pro-apoptotic signalling, which hampers tumour cell growth [30, 31]; and TH1 differentiation, which is critical for generating protective immune responses against cancer [32, 33]. Evidence suggests that tumours can evade immune responses by upregulating TH2 and downmodulating TH1 immune responses; indeed, a TH2 bias correlates with disease progression in certain cancers [34]. Therefore, γδ T-cell production of IFN-γ and TNF-α in response to BCG, M. vaccae and M. obuense immunotherapy could counteract this tumour escape mechanism and restore the TH1 immune responses required to promote anti-cancer immunity.
We also investigated the effects of these mycobacterial preparations on γδ T-cell cytotoxicity against tumour cells. Studies have shown that Vδ2+ γδ T-cells are cytotoxic towards tumour cells from a broad range of haematologic and epithelial cancers [14]. Cytotoxicity is primarily dependent on TCR recognition of phosphoantigens and granzyme/perforin-dependent induction of apoptosis [35]. Also, BCG-specific γδ T-cell lines are cytotoxic towards tumour cells, suggesting that γδ T-cells are cross-reactive [13]. However, the ability of heat-killed mycobacteria to enhance γδ T-cell cytotoxicity has not yet been tested. For the purposes of this study, we used the Burkitt’s lymphoma cell line Daudi as a model tumour target as this cell line is routinely used for investigating γδ T-cell responses against tumour. We found that mycobacteria-stimulated PBMCs contained γδ T-cells with enhanced cytotoxicity against Daudi cells, as shown by increased degranulation. This effect could be explained by upregulation of cytolytic effector molecules; indeed, we found that mycobacteria upregulated γδ T-cell expression of granzyme B, which plays a critical role in γδ T-cell cytotoxicity [36]. Taken together, our data suggest that heat-killed BCG, M. vaccae and M. obuense enhance the cytotoxic activity of γδ T-cells in PBMCs, which may contribute to the anti-tumour properties of these cancer immunotherapies.
Tumour cell lines that are refractory to γδ T-cell killing have been reported; indeed, we found that the A549 tumour cell line failed to induce γδ T-cell degranulation. However, pre-treating A549s with the aminobisphosphonate (ABP) zoledronic acid increased their capacity to induce γδ T-cell degranulation, which confirms previous studies showing that ABPs upregulate phosphoantigen expression in tumours, thus enhancing recognition by Vδ2+ γδ T-cells [37]. We show that PBMCs exposed to heat-killed mycobacteria contain γδ T-cells with enhanced cytotoxic activity against zoledronic acid-treated A549s. This suggests that systemic priming of γδ T-cells with mycobacteria could be combined with local treatment of tumours with ABPs, which would serve to simultaneously increase the visibility of the tumour whilst augmenting the cytotoxic responses of circulating effector cells. The anti-cancer effects of ABPs are currently under investigation in lymphoma, myeloma, prostate cancer and breast cancer [38–40]; however, ABPs are currently administered via intravenous infusions, which poses a number of problems. Firstly, ABPs are rapidly absorbed by the mineral surfaces of bone, thus necessitating high-dose regimens that are often associated with a range of complications including pyrexia, nephrotoxicity and electrolyte abnormalities [41]. Intratumoural administration of ABPs combined with systemic mycobacterial priming may be a more effective treatment regimen that reduces these complications. Secondly, ABPs upregulate phosphoantigens in peripheral blood monocytes, which may render them susceptible to γδ T-cell attack and exhaust γδ T-cell cytotoxic function before they reach the tumour [42]. Similar to ABPs, the chemotherapies etoposide, cisplatin and doxorubicin have also been shown to increase the susceptibility of tumour cell lines to γδ T-cell killing [37]. This suggests there may also be potential in combining certain chemotherapies with BCG, M. vaccae and M. obuense immunotherapy; indeed, a survival benefit was reported in lung cancer patients receiving M. vaccae in combination with platinum-based chemotherapy [6, 7].
In comparing the three different bacterial preparations, only minor differences were observed between them in terms of their activity towards γδ T-cells. Although this suggests that these different preparations each have the potential to elicit comparable anti-tumour responses in γδ T-cells, the potential for differential effects on other immune cells remains to be seen. Moreover, the receptors through which they stimulate mDC1s may differ, which may have bearing on potential combinatorial partners. Further investigations comparing the induced immune responses of these three bacterial preparations are therefore required and are currently underway.
Although γδ T-cell responses to BCG have been previously documented, they have been dependent on viable infection of APCs. For example, Martino et al. [23] reported that DCs pre-treated with live, but not heat-killed, BCG activate γδ T-cells. We found that heat-killed mycobacteria can elicit marked γδ T-cell responses, suggesting there are alternate mechanisms of activation that have yet to be reported. Such mechanisms may be more relevant to cancer immunotherapy since the bulk of reconstituted lyophilised BCG vaccines consist of non-viable bacilli. Furthermore, BCG is slow growing, thus the effects described during live BCG infections are likely to be out-weighed by those elicited by non-viable BCG. Current hypotheses suggest that bacterially infected APCs upregulate expression of γδ T-cell-specific phosphoantigens. In support of this, soluble phosphoantigen-specific γδ TCRs have been shown to selectively bind to BCG-treated but not untreated DCs [43]. Whether these are mycobacteria-derived or endogenous phosphoantigens remains unclear. As shown by Kistowska et al. [44], mycobacterial infections disrupt isoprenoid biosynthesis in APCs, thus causing accumulation of endogenous phosphoantigens. Although mycobacteria-derived and/or endogenous phosphoantigens presented on infected DCs may activate γδ T-cells, their role in γδ T-cell activation by heat-killed preparations of mycobacteria is unclear.
We sought to determine the mechanisms by which heat-killed preparations of mycobacteria induce anti-tumour immune responses in γδ T-cells. Our data suggest that Vδ2+ γδ T-cells are indirectly activated by these mycobacterial preparations via IL-12, IL-1β and TNF-α produced by mDC1s. Parenthetically, it is interesting to note that depleting monocytes from our system resulted in an increase in γδ T-cell activation, which suggests that under certain conditions the bacterial preparations could trigger monocytes to release cytokines that counteract the effects of IL-12, IL-1β and TNF-α. The newly proposed mechanism of activation is contrary to previous reports showing that DCs infected with heat-killed BCG fail to stimulate γδ T-cells [23]. However, in this study cytokine-dependent activation of Vδ2+ γδ T-cells by mDC1s was observed in co-cultures consisting of γδ T-cells and CD4+ cells. Therefore, cytokine production by mDC1s in response to mycobacteria may be dependent on other cells within the CD4+ population. Accordingly, we found that γδ T-cells failed to respond to the heat-killed mycobacteria when co-cultured with purified monocytes or mDC1s (data not shown). This is in keeping with previous reports that pathogen-induced IL-12 production by DCs is dependent on costimulatory signals such as CD40 ligation, which may be supplied by the T-cell component of the CD4+ cell population [45]. The mechanisms underlying mDC1 cytokine production did not fall within the scope of this study and investigations are currently underway.
The observations that heat-killed mycobacteria fail to activate γδ T-cells co-cultured with purified DCs (i.e. in the absence of other CD4+ cells) suggests that phosphoantigen recognition is not involved in the γδ T-cell responses observed here (Figs. 1, 2). It is possible that heat-killing stunts the efficacy of phosphoantigens or perturbs their uptake and processing by mDC1s. Seeing as cancer immunotherapies use either heat-killed or low-viability preparations of mycobacteria, the newly identified mechanism of indirect cytokine priming compared with direct phosphoantigen recognition is more clinically relevant. Furthermore, in terms of generating an anti-tumour γδ T-cell response, the indirect cytokine-mediated priming described here may elicit more favourable responses compared with direct phosphoantigen-mediated activation. Phosphoantigens are target molecules for γδ T-cell cytotoxicity. Therefore, using these target molecules to prime γδ T-cells for immunotherapeutic purposes will cause degranulation, which may exhaust γδ T-cell cytotoxic responses before they encounter tumour. This has bearing on the use of heat-killed instead of live mycobacteria for cancer immunotherapy; indeed, reports have shown that γδ T-cells kill APCs harbouring viable bacterial infections [46]. This also has bearing on immunotherapies that activate γδ T-cells via phosphoantigens, either in situ (i.e. via systemic application of ABPs) or ex vivo (i.e. via adoptive transfer of phosphoantigen-expanded γδ T-cells). In contrast, heat-killed mycobacteria, which indirectly prime γδ T-cells via cytokines from mDC1s, may not exhaust γδ T-cell responses before they encounter tumour. This effect could be exploited to improve immunotherapies so as to avoid exhaustion of γδ T-cells prior to tumour infiltration; for example, by combining systemic administration of mycobacteria with intratumoural administration of ABPs.
In summary, we demonstrate the potential for heat-killed preparations of BCG, M. vaccae and M. obuense to prime an anti-tumour effect in γδ T-cells. Priming is mediated by cytokines from mDC1s and results in production of TH1 cytokines and increased cytotoxicity towards tumour cells. Our data provide a potential explanation for the anti-tumour effects of these mycobacterial preparations in vivo. Furthermore, our data suggest that these immunotherapies can be further developed using combination therapy, for example, combining systemic BCG, M. vaccae and M. obuense with localised ABPs or chemotherapies that augment γδ T-cell susceptibility of target cells. More studies are required to elucidate the full range of effects elicited by these mycobacterial preparations, which are currently underway.
References
Herr HW, Morales A (2008) History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol 179(1):53–56
Maraveyas A, Baban B, Kennard D, Rook GA, Westby M, Grange JM, Lydyard P, Stanford JL, Jones M, Selby P, Dalgleish AG (1999) Possible improved survival of patients with stage IV AJCC melanoma receiving SRL 172 immunotherapy: correlation with induction of increased levels of intracellular interleukin-2 in peripheral blood lymphocytes. Ann Oncol 10(7):817–824
O’Brien ME, Saini A, Smith IE, Webb A, Gregory K, Mendes R, Ryan C, Priest K, Bromelow KV, Palmer RD, Tuckwell N, Kennard DA, Souberbielle BE (2000) A randomized phase II study of SRL172 (Mycobacterium vaccae) combined with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br J Cancer 83(7):853–857
Eaton JD, Perry MJ, Nicholson S, Guckian M, Russell N, Whelan M, Kirby RS (2002) Allogeneic whole-cell vaccine: a phase I/II study in men with hormone-refractory prostate cancer. BJU Int 89(1):19–26
Patel PM, Sim S, O’Donnell DO, Protheroe A, Beirne D, Stanley A, Tourani JM, Khayat D, Hancock B, Vasey P, Dalgleish A, Johnston C, Banks RE, Selby PJ (2008) An evaluation of a preparation of Mycobacterium vaccae (SRL172) as an immunotherapeutic agent in renal cancer. Eur J Cancer 44(2):216–223
O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, Von Pawel J, Reck M, SR-ON-12 Study Group (2004) SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol 15(6):906–914
Stanford JL, Stanford CA, O’Brien ME, Grange JM (2008) Successful immunotherapy with Mycobacterium vaccae in the treatment of adenocarcinoma of the lung. Eur J Cancer 44(2):224–227
Stebbing J, Dalgleish A, Gifford-Moore A, Martin A, Gleeson C, Wilson G, Brunet LR, Grange J, Mudan S (2011) An intra-patient placebo-controlled phase I trial to evaluate the safety and tolerability of intradermal IMM-101 in melanoma. Ann Oncol. doi:10.1093/annonc/mdr363
Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD (2008) Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol 180(9):6044–6053
Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z (2003) Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med 198(3):433–442
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC (2001) Regulation of cutaneous malignancy by gammadelta T cells. Science 294(5542):605–609
Morita CT, Jin C, Sarikonda G, Wang H (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215:59–76
Wang MH, Chen YQ, Gercken J, Ernst M, Böhle A, Flad HD, Ulmer AJ (1993) Specific activation of human peripheral blood gamma/delta + lymphocytes by sonicated antigens of Mycobacterium tuberculosis: role in vitro in killing human bladder carcinoma cell lines. Scand J Immunol 38(3):239–246
Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D (2007) Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol 66(2–3):320–328
Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F (1995) Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol 154(8):3932–3940
Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V (2008) Vgamma9 Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother 57(4):531–539
Lucey DR, Clerici M, Shearer GM (1996) Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev 9(4):532–562
Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW (2002) Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science 295(5563):2255–2258
Garrido F, Algarra I, García-Lora AM (2010) The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible “hard” lesions. Cancer Immunol Immunother 59(10):1601–1606
Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T (2005) Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol 175(4):2144–2151
Wesch D, Marx S, Kabelitz D (1997) Comparative analysis of alpha beta and gamma delta T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol 27(4):952–956
Balaji KN, Boom WH (1998) Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+ alphabeta and gammadelta T cells: role of particulate antigen. Infect Immun 66(1):98–106
Martino A, Casetti R, Sacchi A, Poccia F (2007) Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guérin-infected dendritic cells kill mycobacterial-infected monocytes. J Immunol 179(5):3057–3064
Skeen MJ, Ziegler HK (1995) Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol 154(11):5832–5841
Ueta C, Kawasumi H, Fujiwara H, Miyagawa T, Kida H, Ohmoto Y, Kishimoto S, Tsuyuguchi I (1996) Interleukin-12 activates human gamma delta T cells: synergistic effect of tumor necrosis factor-alpha. Eur J Immunol 26(12):3066–3073
Dieli F, Caccamo N, Meraviglia S, Ivanyi J, Sireci G, Bonanno CT, Ferlazzo V, La Mendola C, Salerno A (2004) Reciprocal stimulation of gammadelta T cells and dendritic cells during the anti-mycobacterial immune response. Eur J Immunol 34(11):3227–3235
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116(16):e74–e80
Wroblewski JM, Bixby DL, Borowski C, Yannelli JR (2001) Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer 33(2–3):181–194
Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J (2001) Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood 98(4):1108–1115
Dormond O, Lejeune FJ, Ruegg C (2002) Modulation of cdk2, cyclin D1, p16INK4a, p21WAF and p27Kip1 expression in endothelial cells by TNF/IFN gamma. Anticancer Res 22(6A):3159–3163
Egwuagu CE, Li W, Yu CR, Che Mei Lin M, Chan CC, Nakamura T, Chepelinsky AB (2006) Interferon-gamma induces regression of epithelial cell carcinoma: critical roles of IRF-1 and ICSBP transcription factors. Oncogene 25(26):3670–3679
Bradley LM, Dalton DK, Croft M (1996) A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol 157(4):1350–1358
Prévost-Blondel A, Roth E, Rosenthal FM, Pircher H (2000) Crucial role of TNF-alpha in CD8 T cell-mediated elimination of 3LL-A9 Lewis lung carcinoma cells in vivo. J Immunol 164(7):3645–3651
Seo N, Hayakawa S, Takigawa M, Tokura Y (2001) Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology 103(4):449–457
Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G (2009) Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol 182(11):7287–7296
Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI (2008) Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 14(13):4232–4240
Mattarollo SR, Kenna T, Nieda M, Nicol AJ (2007) Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother 56(8):1285–1297
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP (2003) Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 102(1):200–206
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC (2007) Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67(15):7450–7457
Santini D, Martini F, Fratto ME, Galluzzo S, Vincenzi B, Agrati C, Turchi F, Piacentini P, Rocci L, Manavalan JS, Tonini G, Poccia F (2009) In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol Immunother 58(1):31–38
Serefoglu EC, Tandogdu Z (2010) Efficacy and safety of zoledronic acid in the treatment of glucocorticoid-induced osteoporosis. Ther Clin Risk Manag 6:219–223
Miyagawa F, Tanaka Y, Yamashita S, Minato N (2001) Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol 166(9):5508–5514
Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW (2008) Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2Vdelta 2 TCR. J Immunol 181(7):4798–4806
Kistowska M, Rossy E, Sansano S, Gober HJ, Landmann R, Mori L, De Libero G (2008) Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR gamma delta cells. Eur J Immunol 38(8):2200–2209
Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C (2000) CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13(4):453–462
Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A (2000) Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol 30(5):1512–1519
Acknowledgments
We thank the Cancer Vaccine Institute for funding this project and Professor John Stanford for supplying the heat-killed preparations of M. vaccae and M. obuense.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fowler, D.W., Copier, J., Wilson, N. et al. Mycobacteria activate γδ T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother 61, 535–547 (2012). https://doi.org/10.1007/s00262-011-1121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1121-4