Abstract
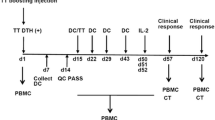
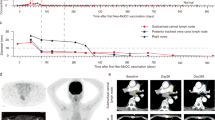
Dendritic cell (DC) vaccine has been used to treat patients with advanced colorectal cancer (CRC). The results of vaccine-induced clinical responses have not always been satisfactory partially because of DC incompetence. In order to evaluate the feasibility of novel mature DCs for therapeutic adjuvants against CRC, we conducted clinical trials with carcinoembryonic antigen (CEA) peptide-loaded DC quickly generated with a combination of OK432 (Streptococcuspyogenes preparation), prostanoid, and interferon-α (OPA-DC). In the ten patients enrolled in this study, the OPA-DC vaccine was well tolerated and administered four times every 2 weeks except for two patients, who were switched to other treatments due to disease progression. Among the eight evaluable patients, one displayed stable disease (SD), while the remaining seven showed progressive disease (PD). In the SD patient, natural killer (NK) cell frequency and cytolytic activity were increased. In the same patient, the frequency of CEA-specific cytotoxic T cells (CTLs) increased stepwise with repetitive vaccinations; however, most of the CTLs exhibited central memory phenotype. In those with PD, NK cells proliferated well regardless of failure of response, whereas CTLs failed to do so. We concluded that the OPA-DC vaccine is well tolerated and has immune-stimulatory capacity in patients with CRC. Additional modulation is needed to attain significant clinical impact.





Similar content being viewed by others
References
Hwang J, Marshall JL (2006) Targeted therapy for colorectal cancer. Curr Opin Investig Drugs 7(12):1062–1066
de Vries IJM, Lesterhuis WJ, Scharenborg NM, Engelen LPH, Ruiter DJ, Gerritsen M-JP, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJA (2003) Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 9(14):5091–5100
Vilella R, Benítez D, Milà J, Lozano M, Vilana R, Pomes J, Tomas X, Costa J, Vilalta A, Malvehy J, Puig S, Mellado B, Martí R, Castel T (2004) Pilot study of treatment of biochemotherapy-refractory stage IV melanoma patients with autologous dendritic cells pulsed with a heterologous melanoma cell line lysate. Cancer Immunol Immunother 53(7):651–658. doi:10.1007/s00262-003-0495-3
Lee AW, Truong T, Bickham K, Fonteneau J-F, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N (2002) A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine 20(Suppl 4):A8–A22
Mbawuike IN, Fujihashi K, DiFabio S, Kawabata S, McGhee JR, Couch RB, Kiyono H (1999) Human interleukin-12 enhances interferon-gamma-producing influenza-specific memory CD8+ cytotoxic T lymphocytes. J Infect Dis 180(5):1477–1486. doi:10.1086/315090
Alli RS, Khar A (2004) Interleukin-12 secreted by mature dendritic cells mediates activation of NK cell function. FEBS Lett 559(1–3):71–76. doi:10.1016/S0014-5793(04)00026-2
Dredge K, Marriott JB, Todryk SM, Dalgleish AG (2002) Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother 51(10):521–531. doi:10.1007/s00262-002-0309-z
Toes RE, Offringa R, Feltkamp MC, Visseren MJ, Schoenberger SP, Melief CJ, Kast WM (1994) Tumor rejection antigens and tumor specific cytotoxic T lymphocytes. Behring Inst Mitt Jul(94):72–86
Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N (2006) Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-alpha as potent immune enhancers. J Immunother 29(1):67–77
Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG (2004) Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int 94(3):412–418. doi:10.1111/j.1464-410X.2004.04922.x
Babatz J, Röllig C, Löbel B, Folprecht G, Haack M, Günther H, Köhne C-H, Ehninger G, Schmitz M, Bornhäuser M (2006) Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer immunology, immunotherapy CII 55(3):268–276. doi:10.1007/s00262-005-0021-x
Liu K-J, Wang C–C, Chen L-T, Cheng A-L, Lin D-T, Wu Y-C, Yu W-L, Hung Y-M, Yang H-Y, Juang S-H, Whang-Peng J (2004) Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin Cancer Res 10(8):2645–2651
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Syrbe U, Siveke J, Hamann A (1999) Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol 21(3):263–285
Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401(6754):708–712. doi:10.1038/44385
Marcusson-Stahl M, Cederbrant K (2003) A flow-cytometric NK-cytotoxicity assay adapted for use in rat repeated dose toxicity studies. Toxicology 193(3):269–279
West E, Morgan R, Scott K, Merrick A, Lubenko A, Pawson D, Selby P, Hatfield P, Prestwich R, Fraser S, Eves D, Anthoney A, Twelves C, Beirne D, Patel P, O’Donnell D, Watt S, Waller M, Dietz A, Robinson P, Melcher A (2009) Clinical grade OK432-activated dendritic cells: in vitro characterization and tracking during intralymphatic delivery. J Immunother 32(1):66–78. doi:10.1097/CJI.0b013e31818be071
Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ (2006) CD4+ CD25+ FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE 1:e129. doi:10.1371/journal.pone.0000129
Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P (2010) Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 33(4):435–441. doi:10.1097/CJI.0b013e3181d32f01
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27(2):186–192. doi:10.1200/JCO.2008.18.7229
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9(2):606–612
Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ (2010) Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res 30(12):5091–5097
Ueda Y, Itoh T, Fuji N, Harada S, Fujiki H, Shimizu K, Shiozaki A, Iwamoto A, Shimizu T, Mazda O, Kimura T, Sonoda Y, Taniwaki M, Yamagishi H (2007) Successful induction of clinically competent dendritic cells from granulocyte colony-stimulating factor-mobilized monocytes for cancer vaccine therapy. Cancer Immunol Immunother CII 56(3):381–389. doi:10.1007/s00262-006-0197-8
Matsuda K, Tsunoda T, Tanaka H, Umano Y, Tanimura H, Nukaya I, Takesako K, Yamaue H (2004) Enhancement of cytotoxic T-lymphocyte responses in patients with gastrointestinal malignancies following vaccination with CEA peptide-pulsed dendritic cells. Cancer Immunol Immunother CII 53(7):609–616. doi:10.1007/s00262-003-0491-7
Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ (2008) Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev 222:256–276. doi:10.1111/j.1600-065X.2008.00617.x
Dauer M, Lam V, Arnold H, Junkmann J, Kiefl R, Bauer C, Schnurr M, Endres S, Eigler A (2008) Combined use of toll-like receptor agonists and prostaglandin E(2) in the FastDC model: rapid generation of human monocyte-derived dendritic cells capable of migration and IL-12p70 production. J Immunol Methods 337(2):97–105. doi:10.1016/j.jim.2008.07.003
Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A (2003) Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol 170(8):4069–4076
Anguille S, Smits ELJM, Cools N, Goossens H, Berneman ZN, Van Tendeloo VFI (2009) Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med 7:109. doi:10.1186/1479-5876-7-109
Avigan DE, Vasir B, George DJ, Oh WK, Atkins MB, McDermott DF, Kantoff PW, Figlin RA, Vasconcelles MJ, Xu Y, Kufe D, Bukowski RM (2007) Phase I/II study of vaccination with electrofused allogeneic dendritic cells/autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother 30(7):749–761. doi:10.1097/CJI.0b013e3180de4ce8
Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM (2008) Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother 31(8):771–780. doi:10.1097/CJI.0b013e3181833818
Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca M-B, Claesson MH, Rosenberg J (2008) Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep 20(6):1305–1311
Trepiakas R, Berntsen A, Hadrup SR, Bjørn J, Geertsen PF, Straten PT, Andersen MH, Pedersen AE, Soleimani A, Lorentzen T, Johansen JS, Svane IM (2010) Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. doi:10.3109/14653241003774045
Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L (2007) Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother 30(7):762–772. doi:10.1097/CJI.0b013e318133451c
Babatz J, Röllig C, Löbel B, Folprecht G, Haack M, Günther H, Köhne C-H, Ehninger G, Schmitz M, Bornhäuser M (2006) Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother 55(3):268–276. doi:10.1007/s00262-005-0021-x
Jinushi M, Takehara T, Kanto T, Tatsumi T, Groh V, Spies T, Miyagi T, Suzuki T, Sasaki Y, Hayashi N (2003) Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol 170(3):1249–1256
Osada T, Clay T, Hobeika A, Lyerly HK, Morse MA (2006) NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol Immunother 55(9):1122–1131. doi:10.1007/s00262-005-0089-3
Shimizu K, Fujii S (2009) DC therapy induces long-term NK reactivity to tumors via host DC. Eur J Immunol 39(2):457–468. doi:10.1002/eji.200838794
Perret R, Ronchese F (2008) Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens 72(3):187–194. doi:10.1111/j.1399-0039.2008.01088.x
Mortarini R, Piris A, Maurichi A, Molla A, Bersani I, Bono A, Bartoli C, Santinami M, Lombardo C, Ravagnani F, Cascinelli N, Parmiani G, Anichini A (2003) Lack of terminally differentiated tumor-specific CD8+ T cells at tumor site in spite of antitumor immunity to self-antigens in human metastatic melanoma. Cancer Res 63(10):2535–2545
Acknowledgments
We are grateful to the members of MTR, especially to the following Drs. Toshiki Yoshimine (Director), Yoshiki Sawa (Director), Akira Myoui (Vice Director), ChunMan Lee, Junji Kawada, Haruki Ide, and Masao Umegaki (Project Managers).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakakibara, M., Kanto, T., Hayakawa, M. et al. Comprehensive immunological analyses of colorectal cancer patients in the phase I/II study of quickly matured dendritic cell vaccine pulsed with carcinoembryonic antigen peptide. Cancer Immunol Immunother 60, 1565–1575 (2011). https://doi.org/10.1007/s00262-011-1051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1051-1