Abstract
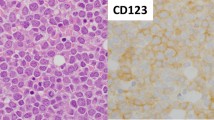
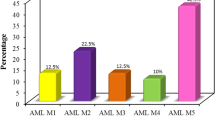
MLAA-34 is a newly identified monocytic leukemia-associated antigen. Previous data indicated that MLAA-34 might be a novel anti-apoptosis factor related closely to carcinogenesis or progression of acute monocytic leukemia. The over-expression of MLAA-34 is intuitively expected to be associated with unfavorable clinical features in acute myeloid leukemia. However, there have been no clinical studies about the prognostic relevance of MLAA-34 expression in human malignancies. This study was done to investigate the clinical relevance of the expression of MLAA-34 in de novo acute myeloid leukemia. In 126 patients with de novo acute myeloid leukemia, the level of MLAA-34 expression and protein expression ratio were determined by using quantitative reverse transcriptase-PCR and western blot, respectively. The results were analyzed with respect to the patients’ clinical features and treatment outcomes. Both MLAA-34 expression rates and expression levels were found to be higher in patients with the French–American–British classification subtype M5, and the expression levels were also higher in patients with a leukocyte number of ≥20 × 109/L and patients with extramedullary disease. In addition, MLAA-34 over-expression (≥median expression) was associated with an unfavorable day 7 response to induction chemotherapy and also associated with a poor survival rate. In multivariate analysis, high MLAA-34 levels was independently associated with a poorer relapse-free survival and overall survival in AML patients. In conclusion, our data indicate that MLAA-34 may be used as a prognostic marker for treatment decision-making in acute monocytic leukemia through validation by further studies.





Similar content being viewed by others
References
Giles FJ, Keating A, Goldstone AH, Avivi I, Willman CL, Kantarjian HM (2002) Acute myeloid leukemia. Hematology 2002(1):73–110. doi:10.1182/asheducation-2002.1.73
Estey E (2006) Acute myeloid leukaemia. Lancet 368(9550):1894–1907
Schaich M, Schlenk RF, Al-Ali HK, Dohner H, Ganser A, Heil G, Illmer T, Krahl R, Krauter J, Sauerland C, Buchner T, Ehninger G (2007) Prognosis of acute myeloid leukemia patients up to 60 years of age exhibiting trisomy 8 within a non-complex karyotype: individual patient data-based meta-analysis of the German acute myeloid leukemia intergroup. Haematologica 92(6):763–770. doi:10.3324/haematol.11100
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK, Goldstone AH, Linch DC (2001) The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom medical research council AML 10 and 12 trials. Blood 98(6):1752–1759. doi:10.1182/blood.V98.6.1752
Madden S, Cook D, Morris J, Gashler A, Sukhatme V, Rauscher F III (1991) Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science 253(5027):1550–1553. doi:10.1126/science.1654597
Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, Nakano T, Suzuki A (2000) Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology 31(5):1080–1085
Greiner J, Li L, Ringhoffer M, Barth TFE, Giannopoulos K, Guillaume P, Ritter G, Wiesneth M, Dohner H, Schmitt M (2005) Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood 106(3):938–945. doi:10.1182/blood-2004-12-4787
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R (2005) The human tumor antigen PRAME is a dominant repressor of retinoic acid. Recept Signal 122(6):835–847
Greiner J, Dohner H, Schmitt M (2006) Cancer vaccines for patients with acute myeloid leukemia—definition of leukemia-associated antigens and current clinical protocols targeting these antigens. Haematologica 91(12):1653–1661
Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, McCarthy LJ (2000) Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, Clinical presentation and management. Leuk Lymphoma 39(1):1–18
Caligiuri MA, Schichman SA, Strout MP, Mrozek K, Baer MR, Frankel SR, Barcos M, Herzig GP, Croce CM, Bloomfield CD (1994) Molecular rearrangement of the ALL-1 gene in acute myeloid leukemia without cytogenetic evidence of 11q23 chromosomal translocations. Cancer Res 54(2):370–373
PaydaŞ S, Zorludemir S, ŞAhin B (2000) Vasculitis and leukemia. Leuk Lymphoma 40(1–2):105–112. doi:10.3109/10428190009054886
Gang C, Wanggang Z, Xingmei C, Fuyang L, Xinping L, Libo Y (2005) Serological identification of immunogenic antigens in acute monocytic leukemia. Leuk Res 29(5):503–509
Zhang P-Y, Zhang W-G, He A-L, Wang J-L, Li W-B (2009) Identification and functional characterization of the novel acute monocytic leukemia associated antigen MLAA-34. Cancer Immunol Immunother 58(2):281–290
Anscher MS (2010) Targeting the TGF-{beta}1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 15(4):350–359. doi:10.1634/theoncologist.2009-S101
Tamm I, Kornblau S, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero D, Tudor G, Qui Y, Monks A (2000) Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res 6(5):1796
Herr I, Debatin K-M (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98(9):2603–2614. doi:10.1182/blood.V98.9.2603
Kawasaki H, Altieri DC, Lu C-D, Toyoda M, Tenjo T, Tanigawa N (1998) Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58(22):5071–5074
Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N (2001) Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 91(11):2026–2032
Niu C, Yan H, Yu T, Sun H-P, Liu J-X, Li X-S, Wu W, Zhang F-Q, Chen Y, Zhou L, Li J-M, Zeng X-Y, Yang R-RO, Yuan M-M, Ren M-Y, Gu F-Y, Cao Q, Gu B-W, Su X-Y, Chen G-Q, Xiong S-M, Zhang T-d, Waxman S, Wang Z-Y, Chen Z, Hu J, Shen Z-X, Chen S-J (1999) Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood 94(10):3315–3324
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PRK, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD (2002) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from cancer and leukemia group B (CALGB 8461). Blood 100(13):4325–4336. doi:10.1182/blood-2002-03-0772
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25(4):402–408
Bernstein S, Brunetto V, Davey F, Wurster-Hill D, Mayer R, Stone R, Schiffer C, Bloomfield C (1996) Acute myeloid leukemia-type chemotherapy for newly diagnosed patients without antecedent cytopenias having myelodysplastic syndrome as defined by French–American–British criteria: a cancer and leukemia group B study. J Clin Oncol 14(9):2486–2494
Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, Head DR, Srivastava DK, Rubnitz JE, Bowman L, Pui C-H, Ribeiro RC (2001) Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution’s experience. Blood 97(12):3727–3732. doi:10.1182/blood.V97.12.3727
San Miguel JF, Vidriales MB, Lopez-Berges C, Diaz-Mediavilla J, Gutierrez N, Canizo C, Ramos F, Calmuntia MJ, Perez JJ, Gonzalez M, Orfao A (2001) Early immunophenotypical evaluation of minimal residual disease in acute myeloid leukemia identifies different patient risk groups and may contribute to postinduction treatment stratification. Blood 98(6):1746–1751. doi:10.1182/blood.V98.6.1746
Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, Sauerland MC, Berdel W, Buchner T, Hiddemann W (2003) Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML cooperative group (AMLCG) 1992 trial. Blood 101(1):64–70. doi:10.1182/blood-2002-02-0532
Gajjar A, Ribeiro R, Hancock M, Rivera G, Mahmoud H, Sandlund J, Crist W, Pui C (1995) Persistence of circulating blasts after 1 week of multiagent chemotherapy confers a poor prognosis in childhood acute lymphoblastic leukemia. Blood 86(4):1292–1295
Browman G, Preisler H, Raza A, Syracuse K, Azarnia N, Benger A, Chervenick P, D’Arrigo P, Doeblin T, Goldberg J, Gottlieb A, Grunwald H, Kirshner J, Larson R, Meyer R, Miller K, Priore R, Stein M, Vogler WR, Walker I, Wilson WEC, Barcos M (1989) Use of the day 6 bone marrow to alter remission induction therapy in patients with acute myeloid leukaemia: a leukemia intergroup study. Br J Haematol 71(4):493–497
Peters WG, Willemze R, Zwaan FE, Colly LP (1988) Day-6 bone marrow aspirate for the prediction of response to remission induction therapy for acute myelogenous leukaemia. Ann Hematol 57(2):91–95
Liso V, Albano F, Pastore D, Carluccio P, Mele G, Lamacchia M, Mestice A, Specchia G (2000) Bone marrow aspirate on the 14th day of induction treatment as a prognostic tool in de novo adult acute myeloid leukemia. Haematologica 85(12):1285–1290
Yang YL, Li XM (2000) The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res 10(3):169–177
Deveraux QL, Reed JC (1999) IAP family proteins—suppressors of apoptosis. Genes Dev 13(3):239–252
Nachmias B, Ashhab Y, Ben-Yehuda D (2004) The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol 14(4):231–243
Zhang H-G, Wang J, Yang X, Hsu H-C, Mountz JD (2004) Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene 23(11):2009–2015
Wrzesień-Kuś A, Smolewski P, Sobczak-Pluta A, Wierzbowska A, Robak T (2004) The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis 9(6):705–715
Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim H-J, Kang HJ, Shin HY, Ahn HS (2007) Expression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood 109(2):471–477. doi:10.1182/blood-2006-07-032557
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China. (No. 30971284).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, J., He, A., Zhang, W. et al. Quantitative assessment of MLAA-34 expression in diagnosis and prognosis of acute monocytic leukemia. Cancer Immunol Immunother 60, 587–597 (2011). https://doi.org/10.1007/s00262-011-0969-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-0969-7