Abstract
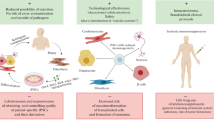
Recent studies have suggested that the core pathways regulating pluripotency in embryonal stem cells bear considerable overlap with oncogenesis. Here, we discuss recent insights into the capacity of the human immune system to target some of the key pluripotency-associated genes. Immunity to these genes is also induced in humans in the context of chemotherapy-induced cell death in patients with germ cell tumors. Immunologic targeting of pathways associated with stemness has implications for both immune regulation of tumor growth as well as emerging regenerative therapies with embryonal stem cells.
Similar content being viewed by others
Abbreviations
- ES:
-
Embryonal stem cells
- MGUS:
-
Monoclonal gammopathy of undetermined significance
- MM:
-
Multiple myeloma
- DC:
-
Dendritic cells
- CSC:
-
Cancer stem cells
- GCT:
-
Germ cell tumor
- TERT:
-
Telomerase reverse transcriptase
References
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Yamanaka S (2009) A fresh look at iPS cells. Cell 137:13–17
Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K (2009) Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460:1145–1148
Krizhanovsky V, Lowe SW (2009) Stem cells: the promises and perils of p53. Nature 460:1085–1086
Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M et al (2009) Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23:2134–2139
Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M (2009) The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460:1136–1139
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40:499–507
Glinsky GV (2008) “Stemness” genomics law governs clinical behavior of human cancer: implications for decision making in disease management. J Clin Oncol 26:2846–2853
Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B (2009) Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun 383:157–162
Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY (2008) Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2:333–344
Gidekel S, Pizov G, Bergman Y, Pikarsky E (2003) Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 4:361–370
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I et al (2009) SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 41:1238–1242
Hussenet T, du Manoir S (2010) SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle 9(8): epub
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM (2006) Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res 66:9339–9344
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D et al (2009) Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113:5412–5417
Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, Davies FE, Drach J, Greipp PR, Kirsch IR et al (2004) Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res 64:1546–1558
Dhodapkar MV, Krasovsky J, Olson K (2002) T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci USA 99:13009–13013
Dhodapkar MV, Krasovsky J, Osman K, Geller MD (2003) Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med 198:1753–1757
Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G et al (2007) Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med 204:831–840
Kim PS, Ahmed R (2010) Features of responding T cells in cancer and chronic infection. Curr Opin Immunol 22:223–230
Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT (2000) Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA 97:4198–4203
Comtesse N, Zippel A, Walle S, Monz D, Backes C, Fischer U, Mayer J, Ludwig N, Hildebrandt A, Keller A et al (2005) Complex humoral immune response against a benign tumor: frequent antibody response against specific antigens as diagnostic targets. Proc Natl Acad Sci USA 102:9601–9606
Blotta S, Tassone P, Prabhala RH, Tagliaferri P, Cervi D, Amin S, Jakubikova J, Tai YT, Podar K, Mitsiades CS et al (2009) Identification of novel antigens with induced immune response in monoclonal gammopathy of undetermined significance. Blood 114:3276–3284
Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, Zebroski H, Dhodapkar MV (2010) Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci USA 107:8718–8723
Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L (2004) OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 28:935–940
Liedtke S, Stephan M, Kogler G (2008) Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem 389:845–850
Berg JS, Goodell MA (2007) An argument against a role for Oct4 in somatic stem cells. Cell Stem Cell 1:359–360
Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R (2007) Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 1:403–415
Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kogler G (2007) Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell 1:364–366
Wegner M, Stolt CC (2005) From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci 28:583–588
Phi JH, Park SH, Kim SK, Paek SH, Kim JH, Lee YJ, Cho BK, Park CK, Lee DH, Wang KC (2008) Sox2 expression in brain tumors: a reflection of the neuroglial differentiation pathway. Am J Surg Pathol 32:103–112
Alcock J, Lowe J, England T, Bath P, Sottile V (2009) Expression of Sox1, Sox2 and Sox9 is maintained in adult human cerebellar cortex. Neurosci Lett 450:114–116
Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE (2010) Expression of pax6 and sox2 in adult olfactory epithelium. J Comp Neurol 518:4395–4418
Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA (2009) Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One 4:e8248
Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C (2010) The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ 17:1345–1353
Kondoh H, Kamachi Y (2010) SOX-partner code for cell specification: regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol 42:391–399
Lauwen MM, Zwaveling S, de Quartel L, Ferreira Mota SC, Grashorn JA, Melief CJ, van der Burg SH, Offringa R (2008) Self-tolerance does not restrict the CD4+ T-helper response against the p53 tumor antigen. Cancer Res 68:893–900
Offringa R, Vierboom MP, van der Burg SH, Erdile L, Melief CJ (2000) p53: a potential target antigen for immunotherapy of cancer. Ann NY Acad Sci 910:223–233 discussion 233–226
Vonderheide RH (2002) Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene 21:674–679
Scanlan MJ, Simpson AJ, Old LJ (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun 4:1
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5:615–625
Dhodapkar MV (2010) Immunity to stemness genes in human cancer. Curr Opin Immunol 22:245–250
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Bindea G, Mlecnik B, Fridman WH, Pages F, Galon J (2010) Natural immunity to cancer in humans. Curr Opin Immunol 22:215–222
Schmitz M, Temme A, Senner V, Ebner R, Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK, Fussel M et al (2007) Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer 96:1293–1301
Ueda R, Ohkusu-Tsukada K, Fusaki N, Soeda A, Kawase T, Kawakami Y, Toda M (2010) Identification of HLA-A2- and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int J Cancer 126(4):919–929
Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F et al (2006) Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res 66:10247–10252
Li Y, Zeng H, Xu RH, Liu B, Li Z (2009) Vaccination with human pluripotent stem cells generates a broad spectrum of immunological and clinical response against colon cancer. Stem Cells 27:3103–3111
Knoepfler PS (2009) Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27:1050–1056
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Shih CC, Forman SJ, Chu P, Slovak M (2007) Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev 16:893–902
Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U et al (2006) Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 24:1433–1440
Qu Q, Shi Y (2009) Neural stem cells in the developing and adult brains. J Cell Physiol 221:5–9
Acknowledgments
MVD is supported by funds from the National Institutes of Health and Leukemia and Lymphoma Society. KMD is supported by funds from the National Institutes of Health, Dana Foundation and Doris Duke Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper is a Focussed Research Review based on a presentation given at the Eighth Annual Meeting of the Association for Cancer Immunotherapy (CIMT), held in Mainz, Germany, May 26–28, 2010.
Rights and permissions
About this article
Cite this article
Dhodapkar, M.V., Dhodapkar, K.M. Spontaneous and therapy-induced immunity to pluripotency genes in humans: clinical implications, opportunities and challenges. Cancer Immunol Immunother 60, 413–418 (2011). https://doi.org/10.1007/s00262-010-0944-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0944-8