Abstract
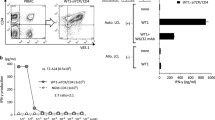
Adoptive cell transfer (ACT), either using rapidly expanded tumor infiltrating lymphocytes or T-cell receptor transduced peripheral blood lymphocytes, can be considered one of the most promising approaches in cancer immunotherapy. ACT results in the repopulation of the host with high frequencies of tumor-specific T cells; however, optimal function of these cells within the tumor micro-environment is required to reach long-term tumor clearance. We and others have shown that ongoing anti-tumor immune responses can be impaired by the expression of ligands, such as PD-L1 (B7-H1) on tumor cells. Such inhibitory molecules can affect T cells at the effector phase via their receptor PD-1. PD-L1/PD-1 interaction has indeed been shown crucial in inducing T-cell anergy and maintaining peripheral tolerance. In order to maximize anti-tumor responses, antibodies that target the PD-1/PD-L1 axis are currently in phase I/II trials. Alternatively, a more refined approach could be the selective targeting of PD-1 in tumor-specific T cells to obtain long-term resistance against PD-1-mediated inhibition. We addressed whether this goal could be achieved by means of retroviral siRNA delivery. Effective siRNA sequences resulting in the reduction of surface PD-1 expression led to improved murine as well as human T-cell immune functions in response to PD-L1 expressing melanoma cells. These data suggest that blockade of PD-1-mediated T-cell inhibition through siRNA forms a promising approach to achieve long-lasting enhancement of tumor-specific T-cell function in adoptive T-cell therapy protocols.




Similar content being viewed by others
Abbreviations
- MFI:
-
Mean fluorescence intensity
- PD-1:
-
Programmed death-1 receptor (CD279)
- PD-L1:
-
Programmed death ligand-1 (B7-H1, CD274)
- PBL:
-
Peripheral blood leukocytes
- TIL:
-
Tumor infiltrating lymphocytes
- TCR:
-
T cell receptor
- mAb:
-
Monoclonal antibody
References
Rosenberg SA, Dudley ME (2009) Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 21:233–240
June CH (2007) Adoptive T cell therapy for cancer in the clinic. J Clin Invest 117:1466–1476. doi:10.1172/JCI32446
Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R (2006) Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 24:5060–5069. doi:10.1200/JCO.2006.07.1100
Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA 99:16168–16173
Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Schallmach E, Kubi A, Shalmon B, Hardan I, Catane R, Segal E, Markel G, Apter S, Nun AB, Kuchuk I, Shimoni A, Nagler A, Schachter J (2009) Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother 32:415–423
Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A (2006) Blockade of PD-L1 (B7–H1) augments human tumor-specific T cell responses in vitro. Int J Cancer 119:317–327
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027–1034
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64:1140–1145
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293–12297
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54:307–314
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi:10.1146/26.021607.090331
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14:3044–3051
Nishimura H, Honjo T (2001) PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 22:265–268
Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T (2005) Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA 102:11823–11828
Weber J (2009) Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 58:823–830. doi:10.1007/s00262-008-0653-8
Hannon GJ (2002) RNA interference. Nature 418:244–251
Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY (1988) Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature 335:271–274
Gajewski TF (1996) B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol 156:465–472
Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H (2002) Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity 17:737–747
Kranz DM, Tonegawa S, Eisen HN (1984) Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA 81:7922–7926
Toebes M, Coccoris M, Bins A, Rodenko B, Gomez R, Nieuwkoop NJ, van de Kasteele W, Rimmelzwaan GF, Haanen JB, Ovaa H, Schumacher TN (2006) Design and use of conditional MHC class I ligands. Nat Med 12:246–251. doi:10.1038/nm1360
Jorritsma A, Gomez-Eerland R, Dokter M, van de Kasteele W, Zoet YM, Doxiadis II, Rufer N, Romero P, Morgan RA, Schumacher TN, Haanen JB (2007) Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood 110:3564–3572. doi:10.1182/blood-2007-02-075010
Gajewski TF, Markiewicz MA, Uyttenhove C (2001) The p815 mastocytoma tumor model. Curr Protoc Immunol. Chapter 20:Unit 20.4. doi:10.1002/0471142735.im2004s43
Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J (2003) B7-h1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol 170:3637–3644
Udaka K, Wiesmuller KH, Kienle S, Jung G, Walden P (1996) Self-MHC-restricted peptides recognized by an alloreactive T lymphocyte clone. J Immunol 157:670–678
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553
Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ (2007) miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129:147–161. doi:S0092-8674(07)00319-4
Uckert W, Schumacher TN (2009) TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol Immunother 58:809–822. doi:10.1007/s00262-008-0649-4
Acknowledgments
This work was supported by the Wilhelm Sander-Stiftung, grant 2005.020.01 and the Dutch Cancer Society (KWF) grant, NKI 2008-3988 to C.B. We thank Bianca Heemskerk for carefully reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borkner, L., Kaiser, A., van de Kasteele, W. et al. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol Immunother 59, 1173–1183 (2010). https://doi.org/10.1007/s00262-010-0842-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0842-0