Abstract
Current therapeutic approaches to treatment of patients with bulky cervical cancer are based on conventional in situ ablative modalities including cisplatin-based chemotherapy and radiation therapy. The 5-year survival of patients with nonresectable disease is dismal. Because over 99% of squamous cervical cancer is caused by persistent infection with an oncogenic strain of human papillomavirus (HPV), particularly type 16 and viral oncoproteins E6 and E7 are functionally required for disease initiation and persistence, HPV-targeted immune strategies present a compelling opportunity in which to demonstrate proof of principle. Sublethal doses of radiation and chemotherapeutic agents have been shown to have synergistic effect in combination with either vaccination against cancer-specific antigens, or with passive transfer of tumor-specific cytotoxic T lymphocytes (CTLs). Here, we explored the combination of low-dose radiation therapy with DNA vaccination with calreticulin (CRT) linked to the mutated form of HPV-16 E7 antigen (E7(detox)), CRT/E7(detox) in the treatment of E7-expressing TC-1 tumors. We observed that TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA vaccination generated significant therapeutic antitumor effects and the highest frequency of E7-specific CD8+ T cells in the tumors and spleens of treated mice. Furthermore, treatment with radiotherapy was shown to render the TC-1 tumor cells more susceptible to lysis by E7-specific CTLs. In addition, we observed that treatment with radiotherapy during the second DNA vaccination generated the highest frequency of E7-specific CD8+ T cells in the tumors and spleens of TC-1 tumor-bearing mice. Finally, TC-1 tumor-bearing mice treated with the chemotherapy in combination with radiation and CRT/E7(detox) DNA vaccination generate significantly enhanced therapeutic antitumor effects. The clinical implications of the study are discussed.
Similar content being viewed by others
Introduction
Despite the fact that it has been possible to screen for cervical cancer for 60 years, this disease is still the second most common cause of cancer death in women worldwide. Persistent infection with an oncogenic strain of human papillomavirus (HPV), most commonly, type 16, causes essentially all squamous cervical cancers. Expression of two viral proteins, E6 and E7, is functionally required for disease initiation and persistence, thereby presenting compelling antigenic targets for therapeutic immunization.
We have developed several targeting strategies for therapeutic DNA vaccines for HPV disease. However, while DNA vaccines present an attractive approach for therapeutic HPV vaccine development because of the ability to engineer exquisite antigen specificity, in humans, to date, potency of vaccination alone has been limited.
We have developed several strategies to enhance potency, by using intracellular targeting strategies to enhance MHC class I/II antigen presentation and processing in dendritic cells (DCs), and by targeting the DNA into DC in vivo by using particle-mediated epidermal delivery (PMED). We have engineered a DNA construct consisting of a pNGVL4a vector containing a mutated form of the HPV16 E7 antigen linked to caltreticulin (CRT). The E7 antigen in this construct has been modified at aa24 and 27, which completely abrogates function while retaining conformation, so that CD8+ T cells elicited by this construct demonstrate antitumor effect against epithelial cells expressing wildtype E7. Calreticulin (CRT) is a 46-kDa Ca2+-binding chaperonin related to the family of heat shock proteins (HSPs) [4, 8, 23]. It promotes assembly of MHC I-peptide complexes delivered into the endoplasmic reticulum by transporters associated with antigen processing (TAP-1 and TAP-2) [28], and also complexes with MHC class I-β2m molecules to aid in antigen presentation [27]. Moreover, CRT and its protein fragment (aa 1-180), vasostatin, have been shown to selectively inhibit vascular endothelial cell proliferation, and to suppress tumor growth. We showed previously that homologous prime-boost vaccination with this construct is effective in enhancing E7-specific CD8+ T cell responses which are capable of eliminating established E7-expressing epithelial tumors, (TC-1) [6, 17]. This vaccine is in phase I testing for patients with early stage, operable (IB1) cervical cancer. (GOG 0702).
More recently, emerging preclinical and clinical evidence suggests that DNA vaccines are a reasonable choice for priming vaccination. In particular, a growing body of evidence suggests that conventional cancer treatment modalities can be combined with immune therapies in a synergistic manner. In fact several clinical studies have employed the combination of radiotherapy with immunotherapy and have been shown to induce tumor-specific and innate immunity [7, 13].
Current clinical strategies for the treatment of (greater than stage IB2) bulky disease primarily involve non-surgical therapies, including radiation and/or cisplatin- based chemotherapy. Sublethal radiation has been shown to increase immunogenicity of solid tumors by several mechanisms, including enhancing the expression of MHC class I molecules [25], as well as increasing expression of adhesion molecules by endothelial cells [10]. These and other phenotypic changes subsequent to radiation render established disease more susceptible to T-cell-mediated lysis [9, 11]. Chemotherapeutic regimens, including cisplatin (CDDP) can also render solid tumors more susceptible to immunologic intervention [11, 20].
In the current study, we explored the combination of radiotherapy with CRT/E7(detox) DNA vaccination in the treatment of E7-expressing TC-1 tumors. We observed that TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA vaccination generated better therapeutic antitumor effects and higher frequency of E7-specific CD8+ T cells compared to treatment with DNA vaccination or radiation alone. Since, in the clinical setting, patients with advanced cervical cancer receive chemoradiation therapy, we also tested the combination of chemoradiation with CRT/E7(detox) vaccination in TC-1 tumor-bearing mice. We found that TC-1 tumor-bearing mice treated with the chemoradiation in conjunction with CRT/E7(detox) DNA generate significantly enhanced therapeutic antitumor effects. The clinical implications of the study are discussed.
Materials and methods
Mice
Female C57BL/6 mice (5–8 weeks old) were obtained from the National Cancer Institute (Frederick, MD) and maintained in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Cell line
Briefly, TC-1 cells were obtained by co-transformation of primary C57BL/6 mouse lung epithelial cells with HPV-16 E6 and E7 and an activated ras oncogene as described previously [18]. Although TC-1 tumor cells are not a true cervical cancer cell line, they serve as a suitable model for the development of therapeutic HPV vaccines since they rely on E6 and E7 for their oncogenicity. The expression of E7 in TC-1 cells has also been characterized previously by He et al. [14].
DNA constructs
The generation of DNA vaccine encoding CRT and E7(detox) has been described previously [17]. Briefly, pNGVL4a-CRT/E7(detox), was generated by PCR amplification of CRT by primers (5′-AAAGTCGACATGCTGCTATCCGTGCCGCTGC -3′ and 5′-GAATTCGTTGTCTGGC-CGCACAATCA-3′) using a human CRT plasmid as a template. The PCR product was digested with SalI/EcoRI and cloned into the SalI/EcoRI sites of pNGVL4a-E7(detox). The accuracy of DNA constructs was confirmed by DNA sequencing.
Particle mediated epidermal delivery of DNA vaccine
DNA-coated gold particles were prepared, and gene gun particle-mediated DNA vaccination was performed, according to a protocol described previously [5]. Gold particles coated with DNA vaccines (1 μg DNA/bullet) were delivered to the shaved abdominal regions of mice by using a helium-driven gene gun (Bio-Rad Laboratories Inc., Hercules, CA) with a discharge pressure of 400 lb/in2. C57BL/6 mice (5 per group) were immunized with 2 μg of the DNA vaccine and received two boost vaccinations with the same dose at 4-day intervals.
In vivo tumor treatment regimens
Sequential radiation and vaccination
To assess in vivo tumor treatment regimens, 9 × 104 TC-1 tumor cells/mouse were injected subcutaneously in the right flank area into 5–8 week-old C57BL/6 mice (5 per group). After 8 days, the mice were divided into groups (5 per group) reflecting different treatment regimens: Group 1 received TC-1 tumor challenge alone; Group 2 was treated with radiation (14 Gy) at day 6; Group 3 was vaccinated with 2 mcg of 4a-CRT/E7 DNA on days 8, 12 and 16; and Group 4 was treated with radiation (14 Gy) on day 6 and then subsequently immunized with 2 mcg of 4a-CRT/E7 DNA on days 8,12, and 16. Mice were monitored twice a week by inspection and palpation. Survival curves on day 63 are shown. Tumor size was monitored by measuring the longest dimension (length) and shortest dimension (width) in a 3-day interval with a dial caliper. Tumor volume was calculated by the following formula: tumor diameter = 0.5× (length + width). At the end of the experiment, mice were killed if tumor size was greater than 2 cm [3].
Radiation combined with vaccination
Groups of C57BL/6 mice (5 per group) were challenged with 9 × 104 TC-1 tumor cells on day 0 and immunized with the 4a-CRT/E7 DNA vaccine on days 8, 12, and 16. Mice were treated with (1) radiotherapy followed by DNA vaccination (RT-DDD), (2) radiotherapy administered on the day of the first vaccination (D-RT-DD), or (3) radiotherapy administered on the day of the second vaccination (DD-RT-D).
Sequential chemoradiation and vaccination
C57BL/6 mice (5 per group) were subcutaneously challenged with 5×104/mouse of TC-1 tumor cells over the left frontal thigh on D0. Tumor-challenged mice were treated with chemoradiation (cisplatin (5 mg/kg) and radiotherapy (14 Gy)) on day 10 and vaccinated with 2 μg 4a-CRT/E7, on days 11, 15, and 19. Cisplatin was intraperitoneally injected at a dose of 5 mg/kg bodyweight. The administered doses were diluted with PBS solution to the required concentration and injected in volumes of 200 μl. Mice treated with vaccination alone were used as a control.
Preparation of single-cell suspensions from tumors
Solid tumors were surgically excised under sterile conditions free of surrounding normal tissue and placed in RPMI 1640 containing 1% antibiotic/antimycotic on ice, and washed with PBS. Tumors were then minced into 1–2-mm pieces and centrifuged at 1,800 rpm for 10 min, and supernantants were removed. After repeating the centrifugation, the tumor fragments were incubated for 45 min to 1 h in a digestion solution (5 ml/g tissue) containing 1 mg/ml trypsin, 1 mg/ml collagenase D, and 0.25 mg/ml DNase in HBSS in an atmosphere of 5% CO2 at 37°C. Pipetting of the samples every 15 min substantially enhanced tissue disruption. DMEM containing 10% FCS and 1% antibiotic/antimycotic was added to the cell suspension to stop the enzymatic activity. To lyse red blood cells, the tissue digests were briefly exposed to cold Tris NH4Cl, washed, and filtered through a 70-μm nylon filter mesh to remove undigested tissue fragments, resulting in a single-cell suspension. The resultant single tumor cell suspensions were washed twice in HBSS (400×g for 10 min), and viable cells purified using single step Ficoll-Hypaque gradients. The gradient interfaces, containing viable tumor cells, lymphocytes and monocytes, were harvested and washed twice with HBSS. The total number of viable cells was then determined using trypan blue dye exclusion and the cells were cryopreserved [31].
Characterization of apoptotic cell death
For characterization of the apoptotic cell death, 2 × 105 TC-1 cells were injected s.c. into the frontal thigh of C57BL/6 mice (5 per group). On day 14, the tumors in the mice were treated with the different doses of radiotherapy; 14 or 36 Gy. Mice were killed 5 days later and the tumors were isolated and single-cell suspensions were prepared as described above. Three hours later, they were stained with PE-conjugated annexin V antibody (BD Pharmingen, San Diego, CA, USA). Flow cytometry analysis was performed to characterize the expression of annexin V positive (apoptotic) cells among the irradiated TC-1 tumor cells. The isotype-matched control antibody was used as the negative control. Flow cytometry analysis was performed using FACSCalibur with CELLQuest software (BD Biosciences, Mountain View, CA, USA).
Intracellular cytokine staining and flow cytometry analysis
Pooled splenocytes from tumor bearing and naïve mice treated with the various treatment regiments were harvested 7 days after the last treatment and incubated for 20 h with 1 μg/ml of E7 peptide containing an MHC class I epitope (aa49–57, RAHYNIVTF) in the presence of GolgiPlug (BD Pharmingen, San Diego, CA, USA) [16]. The stimulated splenocytes were then washed once with FACScan buffer and stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8a (clone 53.6.7). Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Pharmingen, San Diego, CA, USA). Intracellular IFN-γ was stained with FITC-conjugated rat anti-mouse IFN-γ. All antibodies were purchased from BD Pharmingen. Flow cytometry analysis was performed using FACSCalibur with CELLQuest software (BD Biosciences, Mountain View, CA, USA).
In vitro CTL assays
Luciferase-expressing TC-1 cells [15] in medium were seeded into a 24-well round-bottom plate (5 × 104 cells/well). The TC-1 tumor cells were treated with a radiation (RT) dose of 388 rad and incubated in 5% CO2 for 24 h at 37°C. E7-specific cytotoxic T lymphocytes (CTL) from the spleens of tumor-bearing mice immunized with the DNA vaccine served as effector cells and were added in the amount of 1 × 106 cells/well. TC-1 cells expressing luciferase were used as target cells. After incubation, D-luciferin (potassium salt; Xenogen Corp.) was added to each well at 150 μg/ml in media 7–8 min before imaging with the Xenogen IVIS 200 system. CTL-mediated killing was assessed using bioluminescence imaging systems quantitating the decrease of luminescence from baseline.
Statistical analysis
Data presented as mean ± standard error (SE) are representative of at least two different experiments. All P values < 0.05 were considered significant. Statistical analysis was performed using T test for independent samples. Survival distributions for mice in different groups were compared by the Kaplan–Meier curves and by use of the long-rank tests. Analyses were performed using SigmaPlot software (Systat software, Inc.).
Results
TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA vaccination generate the best therapeutic antitumor effects
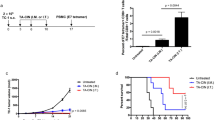
To determine the therapeutic antitumor effects generated by radiotherapy combined with vaccination with DNA encoding CRT/E7(detox), we first challenged groups of C57BL/6 mice (5 per group) with TC-1 tumor cells and then treated them with DNA vaccine alone, radiotherapy alone, or radiotherapy in combination with DNA vaccination as illustrated in Fig. 1a. As shown in Fig. 1b, tumor-bearing mice treated with radiotherapy in combination with CRT/E7(detox) DNA showed significantly reduced tumor size over time as compared to tumor-bearing mice treated with radiotherapy alone or the DNA vaccine alone (P = 0.001). Furthermore, tumor-bearing mice treated with radiotherapy in combination with CRT/E7(detox) DNA showed improved survival compared to tumor-bearing mice treated with radiotherapy alone or the DNA vaccine alone (P = 0.003) (Fig. 1c). Thus, our data indicate that the treatment regimen using radiotherapy in combination with CRT/E7(detox) DNA produces the best therapeutic antitumor effects and long-term survival in TC-1 tumor-bearing mice.
In vivo tumor treatment experiments. a Schematic diagram of the different treatment regimens of radiotherapy and/or DNA vaccine, including radiotherapy alone (RT), DNA vaccination alone (4a-CRT/E7d) or radiotherapy followed by DNA vaccination (RT-4a-CRT/E7d). Groups of C57BL/6 mice (5 per group) were subcutaneously challenged with 9 × 104/mouse of TC-1 tumor cells over the left frontal thigh on D0. Tumor challenged mice were treated with radiotherapy and/or DNA encoding 4a-CRT/E7d as indicated in the time line. Local radiotherapy (825.13/min = dose 1.7 min) 14 Gy was administered on D6. DNA was administered via gene gun in the amount of 2 µg/mouse three times with 4-day intervals on D8, D12 and D16. Untreated TC-1 tumor-bearing mice were used as a control. Tumor size and volume was measured as described in the “Materials and methods”. b Line graph depicting the tumor diameter in TC-1 tumor bearing mice treated with the different treatment regimens (mean ± SE). c Kaplan and Meier survival analysis of TC-1 tumor challenged mice treated with the different treatment regimens. Data shown are representative of two experiments performed
Treatment of TC-1 tumor cells from tumor-bearing mice with radiotherapy enhances the apoptotic tumor cell death
In order to determine the effect of radiotherapy on TC-1 tumor cells in tumor-bearing mice, we isolated TC-1 tumor cells from tumor-bearing mice treated with different doses of radiation. The cells were then characterized for apoptotic cell death using annexin V staining. As shown in Fig. 2, we observed that TC-1 tumor cells treated with the higher doses of radiotherapy demonstrated a greater degree of apoptotic tumor cell death compared to control untreated TC-1 tumor cells. Thus, our data indicate that radiotherapy enhances the apoptotic tumor cell death in TC-1 tumors.
Flow cytometry analysis to demonstrate the expression of annexin V on radiotherapy treated TC-1 tumor cells. C57BL/6 mice (5 per group) were injected s.c. into the frontal thigh with 2 × 105 TC-1 cells on D0. After 14 days, tumor-bearing mice were treated with different doses of radiotherapy; 14 or 36 Gy. Mice were sacrificed 5 days later and the tumor cells were isolated. The cells were then stained with PE-conjugated annexin V antibody (BD, San Diego) to detect the expression of annexin V. Flow cytometry analysis was performed to characterize the expression of annexin V positive (apoptotic) cells among the irradiated TC-1 tumor cells. The isotype-matched control antibody was used as the negative control (black profile)
TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA generate highest frequency of E7-specific CD8+ T cells
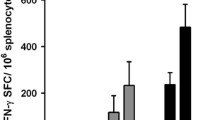
In order to determine the E7-specific CD8+ T cell immune response in tumor-bearing mice treated with radiotherapy in combination with the CRT/E7(detox) DNA vaccine, we first challenged groups of C57BL/6 mice (5 per group) with TC-1 tumor cells and then treated them with DNA vaccine alone, radiotherapy alone, or radiotherapy in combination with DNA vaccination as illustrated in Fig. 1a. Seven days after the last treatment, we harvested splenocytes from vaccinated mice and characterized the presence of E7-specific CD8+ T cells in treated mice using intracellular cytokine staining for IFN-γ followed by flow cytometry analysis. As shown in Fig. 3, tumor-bearing mice that were treated with radiotherapy in combination with CRT/E7(detox) DNA generated a significantly higher number of E7-specific CD8+ T cells compared to tumor-bearing mice that were administered CRT/E7(detox) DNA alone, or radiotherapy alone (P = 0.058). These results indicate that treatment of tumor-bearing mice with radiotherapy in combination with CRT/E7(detox) DNA leads to the strongest E7-specific CD8+ T cell immune response.
Intracellular cytokine staining followed by flow cytometry analysis to determine the number of E7-specific CD8+ T cells in tumor-bearing mice treated with radiotherapy and/or DNA vaccine. Groups of C57BL/6 mice (5 per group) were challenged with TC-1 tumor cells and treated with radiotherapy and/or DNA as illustrated in Fig. 1a. After 23 days tumor challenge, splenocytes from mice were harvested and stained for CD8 and intracellular IFN-γ and then characterized for E7-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. a Representative data of intracellular cytokine stain followed by flow cytometry analysis showing the number of E7-specific IFN-γ+ CD8+ T cells in mice treated with radiotherapy and/or DNA vaccine (right upper quadrant). b Bar graph depicting the numbers of E7-specific IFN-γ-secreting CD8+ T cells per 3×105 pooled splenocytes (mean ± SE). Data shown are representative of two experiments performed
TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA generate highest frequency of E7-specific CD8+ T cells in the tumor-infiltrating lymphocytes
In order to determine the E7-specific CD8+ T cell immune response in the tumor infiltrating lymphocytes (TILs) of tumor-bearing mice treated with radiotherapy in combination with the CRT/E7(detox) DNA vaccine, we first challenged groups of C57BL/6 mice (5 per group) with TC-1 tumor cells and then treated them with DNA vaccine alone, radiotherapy alone, or radiotherapy in combination with DNA vaccination as illustrated in Fig. 1a. Seven days after the last treatment, we harvested the TILs from vaccinated mice and characterized the presence of E7-specific CD8+ T cells in treated mice using intracellular cytokine staining for IFN-γ followed by flow cytometry analysis. As shown in Fig. 4, tumor-bearing mice that were treated with radiotherapy in combination with CRT/E7(detox) DNA generated a significantly higher number of E7-specific CD8+ T cells in the TILs compared to tumor-bearing mice that were administered CRT/E7(detox) DNA alone or radiotherapy alone (P = 0.006). These results indicate that treatment with radiotherapy in combination with CRT/E7(detox) DNA leads to the strongest E7-specific CD8+ T cell immune response in the TILs of tumor-bearing mice.
Intracellular cytokine staining followed by flow cytometry analysis to determine the number of E7-specific CD8+ TILs in tumor-bearing mice treated with radiotherapy and/or DNA vaccine. Groups of C57BL/6 mice (5 per group) were challenged with TC-1 tumor cells and treated with radiotherapy and/or DNA as illustrated in Fig. 1a. After 23 days tumor challenge, tumor-infiltrating lymphocytes from mice were harvested and stained for CD8 and intracellular IFN-γ and then characterized for E7-specific CD8+ TILs using intracellular IFN-γ staining followed by flow cytometry analysis. a Representative data of intracellular cytokine stain followed by flow cytometry analysis showing the number of E7-specific IFN-γ+ CD8+ TILs in mice treated with radiotherapy and/or DNA vaccine (right upper quadrant). b Bar graph depicting the numbers of E7-specific IFN-γ-secreting CD8+ TILs per 1 × 105 TILs (mean ± SE). Data shown are representative of two experiments performed
Treatment with radiotherapy renders the TC-1 tumor cells more susceptible to lysis by E7-specific CTLs
In order to determine if treatment of TC-1 tumor cells with radiotherapy will render the tumor cell more susceptible to E7-specific T cell-mediated killing, we performed a cytotoxicity assay using luciferase-expressing TC-1 tumor cells. TC-1 tumor cells were treated with radiotherapy alone, radiotherapy and E7-specific cytotoxic T cells (CTL), or treated with E7-specific CTLs alone. Untreated TC-1 tumor cells were used as a control. The CTL-mediated killing of the TC-1 tumor cells in each well was monitored using bioluminescent imaging systems. The degree of CTL-mediated killing of the tumor cells was indicated by the decrease of luminescence activity. As shown in Fig. 5, the lowest luciferase activity was observed in the wells incubated with radiotherapy and E7-specific CTLs as compared to the wells incubated with radiotherapy alone or E7-specific CTLs alone (P = 0.002). Thus, our data suggest that the TC-1 tumor cells treated with radiotherapy increased the susceptibility of the tumor cells for lysis by the E7-specific cytotoxic T cells.
In vitro cytotoxicity assay. Luciferase-expressing TC-1 tumor cells were a added to 24-well plates at a dose of 1 × 105/well. TC-1/luc tumor cells were a untreated, b irradiated (388 rad), c irradiated and treated with 1.3 × 106 E7-specific cytotoxic T cells (CTL) or d treated with 1 × 106 E7-specific CTLs alone for 4.5 h. The degree of CTL-mediated killing of the tumor cells was indicated by the decrease of luminescence activity using the IVIS luminescence imaging system series 200. Bioluminescence signals were acquired for one minute. a Representative luminescence images of 24-well plates showing lysis of the tumor cells. b Bar graph depicting the quantification of luminescence intensity in tumor cells treated with radiotherapy and/or E7-specific cytotoxic T cells (mean ± SE)
Treatment with radiotherapy during the second DNA vaccination generates highest frequency of E7-specific CD8+ T cells in the tumors and spleens of TC-1 tumor-bearing mice
In order to determine the E7-specific CD8+ T cell immune response in the tumors and spleens of tumor-bearing mice treated with radiotherapy in combination with the CRT/E7(detox) DNA vaccine, we first challenged groups of C57BL/6 mice (5 per group) with TC-1 tumor cells and then treated them with radiotherapy in combination with DNA vaccination using different regimens as illustrated in Fig. 6a. Seven days after the last treatment, we harvested the splenocytes and the TILs from vaccinated mice and characterized the presence of E7-specific CD8+ T cells in treated mice using intracellular cytokine staining for IFN-γ followed by flow cytometry analysis. As shown in Fig. 6b, tumor-bearing mice that were treated with radiotherapy during the second CRT/E7(detox) DNA vaccination generated a significantly higher number of E7-specific CD8+ T cells in the splenocytes (P = 0.001) (Fig. 6c) and TILs (P = 0.015) (Fig. 6d) compared to tumor-bearing mice that were treated with radiotherapy during the first DNA vaccination or before DNA vaccination. These results indicate that treatment with radiotherapy during the second CRT/E7(detox) DNA vaccination leads to the strongest E7-specific CD8+ T cell immune response in the spleens and tumors of tumor-bearing mice. We also followed the tumor growth and survival in tumor-bearing mice treated with the different regimens. Interestingly, we observed that tumor-bearing mice treated with radiation before the first DNA vaccination demonstrated significantly lower tumor size compared to tumor-bearing mice treated with radiotherapy during the first or second DNA vaccination (See Supplementary Fig. 1).
Intracellular cytokine staining followed by flow cytometry analysis to determine the number of E7-specific CD8+ T cells in the spleen and tumors of tumor-bearing mice treated with radiotherapy and/or DNA vaccine. a Schematic diagram of the different treatment regimens of radiotherapy and/or DNA vaccine, including radiotherapy followed by DNA vaccination (RT-DDD), radiotherapy administered on the day of the first vaccination (D-RT-DD) or radiotherapy administered on the day of the second vaccination (DD-RT-D). Groups of C57BL/6 mice (5 per group) were challenged with TC-1 tumor cells on day 0 and immunized with the 4a-CRT/E7d DNA vaccine on days 8, 12 and 16. Mice were treated with radiotherapy as illustrated. After 23 days tumor challenge, splenocytes and the TILs from mice were harvested and stained for CD8 and intracellular IFN-γ and then characterized for E7-specific CD8+ T cells using intracellular IFN-γ staining followed by flow cytometry analysis. b Representative data of intracellular cytokine stain followed by flow cytometry analysis showing the number of E7-specific IFN-γ+ CD8+ T cells in the various groups (right upper quadrant). c, d Bar graph depicting the numbers of (c) E7-specific IFN-γ-secreting CD8+ T cells per 3 × 105 pooled splenocytes and d E7-specific IFN-γ-secreting CD8+ T cells per 15 × 103 TILs in the tumor (mean ± SE). Data shown are representative of two experiments performed
In order to determine the E7-specific CD8+ T cell immune response in naïve mice treated with the similar regimens of radiotherapy in combination with the CRT/E7(detox) DNA vaccine, we treated naïve mice using the regimen similar to the one described above (See Supplementary Fig. 2a). We then harvested the splenocytes from vaccinated mice 7 days after the last treatment, and characterized the presence of E7-specific CD8+ T cells using intracellular cytokine staining for IFN-γ followed by flow cytometry analysis. We observed that mice that were treated with radiotherapy during the second DNA vaccination generated a significantly higher number of E7-specific CD8+ T cells compared to mice that were administered CRT/E7(detox) DNA alone (P = 0.039) (Supplementary Fig. 2b and c). Taken together, our results indicate that mice treated with radiotherapy during the second CRT/E7(detox) DNA vaccination demonstrate the strongest E7-specific CD8+ T cell immune response.
TC-1 tumor-bearing mice treated with the combination of radiotherapy and chemotherapy and vaccinated with CRT/E7(detox) DNA generate potent therapeutic antitumor effects
The standard therapy for cervical cancer employs chemoradiation using cisplatin in combination with radiotherapy. Thus, in order to determine the therapeutic antitumor effects generated by the combination of radiotherapy and chemotherapy in CRT/E7(detox) DNA vaccinated mice, we first challenged groups of C57BL/6 mice (5 per group) with TC-1 tumor cells and then treated them with the combination of radiotherapy and chemotherapy using cisplatin. One day later, mice were vaccinated with the CRT/E7(detox) DNA vaccine 3 times with 4-day intervals as illustrated in Fig. 7a. As shown in Fig. 7b, tumor-bearing mice treated with the combination of radiotherapy and chemotherapy and vaccination with CRT/E7(detox) DNA showed significantly reduced tumor size over time as compared to tumor-bearing mice treated with radiotherapy and chemotherapy alone or the DNA vaccine alone (P = 0.009). Furthermore, tumor-bearing mice treated with the combination of radiotherapy and chemotherapy and vaccination with CRT/E7(detox) DNA showed improved survival compared to tumor-bearing mice treated with radiotherapy and chemotherapy alone or the DNA vaccine alone (P = 0.002) (Fig. 7c). We also found that the antitumor effects generated by radiotherapy with CRT/E7(detox) DNA vaccination were quite comparable with the antitumor effects generated by chemoradiation (cisplatin + radiation) with CRT/E7(detox) DNA vaccination (data not shown). Thus, our data indicate that the treatment regimen using chemoradiation in combination with CRT/E7(detox) DNA produces potent therapeutic antitumor effects and long-term survival in TC-1 tumor-bearing mice.
In vivo tumor treatment experiments in mice treated with chemoradiation and/or CRT/E7 DNA vaccination. a Schematic diagram of the treatment regimen of cisplatin and radiation (chemoradiation) and DNA vaccination. C57BL/6 mice (5 per group) were subcutaneously challenged with 5 × 104/mouse of TC-1 tumor cells over the left frontal thigh on D0. Tumor challenged mice were treated with chemoradiation (cisplatin administered intraperitoneally at a dose of 5 mg/kg) and radiotherapy (14 Gy)) on day 10 and vaccinated with or without CRT/E7 DNA as indicated in the time line. DNA was administered via gene gun in the amount of 2 µg/mouse three times with 4-day intervals on D11, D15 and D19. Mice treated with DNA vaccination alone were used as a control. b Line graph depicting the tumor volume in TC-1 tumor bearing mice treated with chemoradiation and vaccinated with the DNA vaccine (mean ± SE). c Kaplan and Meier survival analysis of TC-1 tumor challenged mice treated with chemoradiation and vaccinated with the DNA vaccine. Data shown are representative of two experiments performed
Discussion
In the current study, we observed that TC-1 tumor-bearing mice treated with radiotherapy combined with CRT/E7(detox) DNA vaccination generated higher frequency of E7-specific CD8+ T cells in the spleen and in TILs, resulting in better therapeutic antitumor effects and in treated mice. We also showed that CRT/E7(detox) DNA vaccination can also be used in conjunction with chemoradiation to significantly enhance the therapeutic antitumor effects in tumor-bearing mice. Thus, the encouraging preclinical data suggests that CRT/E7(detox) DNA may potentially be used in conjunction with conventional chemoradiation therapy in patients with advanced stage cervical cancer.
The fact that HPV is an etiological factor for cervical cancer has created an opportunity to control HPV-associated cervical cancer by vaccination against HPV. The prophylactic vaccines, while effective, present essentially the same barriers to uptake as those for screening and early treatment, and are unlikely to significantly change the burden of disease in the near future [21, 26]. In the meantime, because the expression of HPV viral antigens, E6 and/or E7 is necessary to maintain the malignant phenotype, from an immunologic standpoint, HPV-associated disease is a compelling target for the development of therapeutic vaccines. Thus, in order to accelerate the control of cervical cancer and treat currently infected patients, it is important to continue the development of therapeutic vaccines against HPV.
In the current study, we have used low-dose radiation (14 Gy) in combination with CRT/E7(detox) DNA vaccination to generate E7-specific CD8+ T cell immune responses and therapeutic antitumor effects and in treated mice. A recent study by Ye et al. has characterized the dose effect of a range of irradiation doses in mice with established, 7-mm-diameter TC-1 tumors, ranging from 10–50 Gy [30]. These authors also found that irradiation increased the sensitivity of tumor to therapeutic vaccination, and that doses of irradiation could be decreased and still remain effective when used in combination with protein vaccines in combination with CpG adjuvant in mice. Thus, our results are consistent with previous studies.
In our study, we showed that radiotherapy followed by CRT/E7(detox) DNA vaccination generate the best therapeutic antitumor effects compared to radiotherapy alone or DNA vaccination alone (See Fig. 1). Previous studies have shown that conventional cancer treatment modalities, including chemotherapy and radiation, can have immunoadjuvant effects [1, 2, 12, 24, 32]. However, the mechanisms by which these synergies occur are incompletely understood. Low-dose irradiation may enhance the immunogenicity and tumor infiltration with antigen-specific CD8+ T cells of E7-targeted vaccination in tumor-bearing hosts. In addition, radiation may also phenotypically modulate the tumor by upregulation of chemokines, MHC molecules, tumor-associated antigens, costimulatory molecules, thus making it easier for tumor specific T-cells to traffic to and recognize tumor [19, 22]. This may lower the threshold for immune mediated killing, which can increase the inflammatory infiltrate. Thus our data are consistent with the notion that tumor cell death induced by conventional cancer treatment modalities may have immunoadjuvant effects.
We observed the tumor-bearing mice that were treated with radiotherapy before CRT/E7(detox) DNA vaccination (RT-DDD) demonstrated the lowest tumor load compared to tumor-bearing mice treated with radiotherapy during the first DNA vaccination (D-RT-DD) or second DNA vaccination (DD-RT-D) (See Supplementary Fig. 1). Thus, our data suggest that there is no direct correlation between the tumor burden and the E7-specific CD8+ T cell immune responses in treated mice. A possible explanation for the significant therapeutic effect generated by treatment with radiation before DNA vaccination may be due to significant control of tumor by radiation during the early stages of tumor growth. The observed enhancement in the E7-specifc CD8+ T cell immune responses in mice treated with radiotherapy during the second DNA vaccination (DD-RT-D) may be related to the amount of the E7 tumor antigen released in treated mice following radiotherapy, although we cannot exclude other possibilities.
The IFN-γ production in the tumor microenvironment may be important for the observed enhanced antitumor effect generated by radiotherapy in conjunction with immunotherapy. Recently, Lugade et al. have demonstrated that radiation is capable of inducing IFN-γ production in the tumor microenvironment [19]. The IFN-γ present in the tumor microenvironment may lead to the upregulation of MHC class I in the tumor cells, resulting in increased tumor cell target recognition by the antigen-specific CD8+ T cells and increased T cell infiltration. All these factors may potentially contribute to the radiation-induced enhancement of antitumor immunity.
Our study demonstrated that chemoradiation combined with CRT/E7(detox) DNA vaccination generated significantly potent therapeutic antitumor effects against TC-1 tumors (See Fig. 7). We have recently demonstrated that pretreatment with cisplatin enhanced E7-specific CD8+ T cell-mediated antitumor immunity induced by CRT/E7(detox) DNA vaccination [29]. Pretreatment of the tumor with cisplatin was shown to lead to apoptosis of the tumor cells, causing the uptake of E7 antigen by the APCs and activation of E7-specific CD8+ T cells. Similarly, in the current study, we have shown that radiation leads to an increase in the apoptosis of the tumor cells, resulting in increased number of E7-specific CD8+ T cell precursors. Because the standard therapy for advanced cervical cancer employs chemoradiation, our data support the notion that the conventional chemoradiation therapy may be combined with HPV DNA vaccination to further improve antitumor effects and survival of patients with advanced cervical cancer.
The DNA vaccine we evaluated was engineered to improve immunogenicity by two strategies; by enhancing MHC Class I processing and presentation via linkage of antigen to CRT, a chaperonin, and by using PMED to optimize delivery to relevant dendritic cells. While we are in the process of testing this construct clinically in a patient cohort with minimal residual disease, the finding that radiotherapy could improve the immunogenicity and efficacy of targeted vaccination would be potentially useful for patients with inoperable disease.
References
Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N et al (2006) Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12:878–887
Arlen PM, Pazdur M, Skarupa L, Rauckhorst M, Gulley JL (2006) A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229). Clin Breast Cancer 7:176–179
Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI (2007) Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res 13:341–349
Basu S, Srivastava PK (1999) Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med 189:797–802
Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM et al (2000) Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res 60:1035–1042
Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M et al (2001) Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest 108:669–678
Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y et al (2005) Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 28:129–135
Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M (1995) Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. J Biol Chem 270:17011–17016
Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64:7985–7994
Gaugler MH, Squiban C, van der Meeren A, Bertho JM, Vandamme M, Mouthon MA (1997) Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol 72:201–209
Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C et al (2006) Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res 12:1897–1905
Gribben JG, Ryan DP, Boyajian R, Urban RG, Hedley ML, Beach K et al (2005) Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res 11:4430–4436
Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P et al (2005) Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 11:3353–3362
He Z, Wlazlo AP, Kowalczyk DW, Cheng J, Xiang ZQ, Giles-Davis W et al (2000) Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology 270:146–161
Huang B, Mao CP, Peng S, He L, Hung CF, Wu TC (2007) Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine 25:7824–7831
Ji H, Wang TL, Chen CH, Pai SI, Hung CF, Lin KY et al (1999) Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther 10:2727–2740
Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK et al (2004) Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther 11:1011–1018
Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM et al (1996) Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 56:21–26
Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM (2008) Radiation-Induced IFN-{gamma} Production within the Tumor Microenvironment Influences Antitumor Immunity. J Immunol 180:3132–3139
Matsuzaki I, Suzuki H, Kitamura M, Minamiya Y, Kawai H, Ogawa J (2000) Cisplatin induces fas expression in esophageal cancer cell lines and enhanced cytotoxicity in combination with LAK cells. Oncology 59:336–343
Monie A, Hung CF, Wu TC (2007) Preventive and therapeutic HPV vaccines. Curr Opin Investig Drugs 8:1038–1050
Moriconi F, Christiansen H, Raddatz D, Dudas J, Hermann RM, Rave-Frank M et al (2008) Effect of radiation on gene expression of rat liver chemokines: in vivo and in vitro studies. Radiat Res 169:162–169
Nash PD, Opas M, Michalak M (1994) Calreticulin: not just another calcium-binding protein. Mol Cell Biochem 135:71–78
Noguchi M, Itoh K, Yao A, Mine T, Yamada A, Obata Y et al (2005) Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24 + HRPC patients. Prostate 63:1–12
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK et al (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271
Roden R, Wu TC (2003) Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2:495–516
Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P (1996) Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 5:103–114
Spee P, Neefjes J (1997) TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur J Immunol 27:2441–2449
Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D et al (2008) Pretreatment with Cisplatin Enhances E7-Specific CD8+ T-Cell-Mediated Antitumor Immunity Induced by DNA Vaccination. Clin Cancer Res 14:3185–3192
Ye GW, Park JB, Park YJ, Choi YS, Sin JI (2007) Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol Ther 15:1564–1570
Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A et al (2007) Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 204:49–55
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8:59–73
Acknowledgments
This work was supported by the Flight Attendant Medical Research Institute and National Cancer Institute SPORE programs (P50 CA098252 and P50 CA96784-06) and the 1 RO1 CA114425-01.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tseng, CW., Trimble, C., Zeng, Q. et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother 58, 737–748 (2009). https://doi.org/10.1007/s00262-008-0596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0596-0