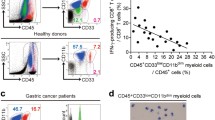
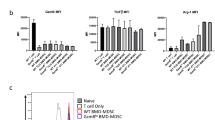
Abstract
An elevated number of Gr-1+CD11b+ myeloid-derived suppression cells (MDSCs) has been described in mice and human bearing tumor and associated with immune suppression. Arginase I production by MDSCs in the tumor environment may be a central mechanism for immunosuppression and tumor evasion. In this study and before, we found that Gr-1+CD11b+ MDSCs from ascites and spleen of mice bearing ovarian 18D carcinoma express a high level of PD-1, CTLA-4, B7-H1 and CD80 while other co-stimulatory molecules, namely CD40, B7-DC and CD86 are not detected. Further studies showed that PD-1 and CTLA-4 on the Gr-1+CD11b+ MDSCs regulated the activity and expression of arginase I. The blockage and silencing of PD-1, CTLA-4 or both PD-1 and CTLA4 molecules could significantly reduce arginase I activity and expression induced with tumor-associated factor. Similar results were also observed while their ligands B7-H1 and/or CD80 were blocked or silenced. Furthermore, CD80 deficiency also decreased the arginase I expression and activity. Antibody blockade or silencing of PD-1, CTLA-4 or both reduced the suppressive potential of PD-1+CTLA-4+MDSCs. Blockade of PD-1, CTLA-4 or both also slowed tumor growth and improved the survival rate of tumor-bearing mice. Thus, there may exist a coinhibitory and costimulatory molecules-based immuno-regulating wet among MDSCs.







Similar content being viewed by others
References
Young MR, Wright MA, Matthews JP, Malik I, Prechel M (1996) Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J Immunol 156:1916–1922
Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T (1996) Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA 93:13119–13124
Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R (1996) Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol 26:1308–1313
Fu YX, Watson GA, Kasahara M, Lopez DM (1991) The role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. I. Induction of regulatory macrophages in normal mice by the in vivo administration of rGM-CSF. J Immunol 146:783–789
Bronte V, Serafini P, Apolloni E, Zanovello P (2001) Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother 24:431–446
Melani C, Chiodoni C, Forni G, Colombo MP (2003) Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB–2 transgenic BALB/c mice suppresses immune reactivity. Blood 102:2138–2145
Kusmartsev S, Gabrilovich DI (2003) Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol 74:186–196
Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V (2004) Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother 53:64–72
Frey AB (2006) Myeloid suppressor cells regulate the adaptive immune response to cancer. J Clin Invest 116:2587–2590
Seung LP, Rowley DA, Dubey P, Schreiber H (1995) Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA 92:6254–6258
Allez M, Mayer L (2004) Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis 10:666–676
Mills SL, Catania KC (2004) Identification of retinal neurons in a regressive rodent eye (the naked mole-rat). Vis Neurosci 21:107–117
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 168:689–695
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX–2 in lung carcinoma. J Exp Med 202:931–939
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64:5839–5849
Morris SM Jr (2004) Recent advances in arginine metabolism. Curr Opin Clin Nutr Metab Care 7:45–51
Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF (2000) Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 21:585–591
Tseng JC, Hurtado A, Yee H, Levin B, Boivin C, Benet M, Blank SV, Pellicer A, Meruelo D (2004) Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res 64:6684–6692
Corraliza IM, Campo ML, Soler G, Modolell M (1994) Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174:231–235
Yang R, Cai Z, Zhang Y, WHt Yutzy, Roby KF, Roden RB (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+myeloid cells. Cancer Res 66:6807–6815
Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW (1996) Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science 272:1170–1173
Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T (2001) PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA 98:13866–13871
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774
Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P (2005) Studying the immunosuppressive role of indoleamine 2, 3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int 18:95–100
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3:541–547
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270:985–988
Bluestone JA (1997) Is CTLA-4 a master switch for peripheral T cell tolerance? J Immunol 158:1989–1993
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548
Chitnis T, Najafian N, Abdallah KA, Dong V, Yagita H, Sayegh MH, Khoury SJ (2001) CD28-independent induction of experimental autoimmune encephalomyelitis. J Clin Invest 107:575–583
Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA (1996) CTLA-4: a negative regulator of autoimmune disease. J Exp Med 184:783–788
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2:261–268
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8:765–772
Porembska Z, Luboinski G, Chrzanowska A, Mielczarek M, Magnuska J, Baranczyk-Kuzma A (2003) Arginase in patients with breast cancer. Clin Chim Acta 328:105–111
Porembska Z, Skwarek A, Mielczarek M, Baranczyk-Kuzma A (2002) Serum arginase activity in postsurgical monitoring of patients with colorectal carcinoma. Cancer 94:2930–2934
Gokmen SS, Aygit AC, Ayhan MS, Yorulmaz F, Gulen S (2001) Significance of arginase and ornithine in malignant tumors of the human skin. J Lab Clin Med 137:340–344
Keskinege A, Elgun S, Yilmaz E (2001) Possible implications of arginase and diamine oxidase in prostatic carcinoma. Cancer Detect Prev 25:76–79
Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB (2003) Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol 170:5064–5074
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by Nankai University grant, NSFC grant “30771967”, “985” grant,The Ministry of Science and Technology grant “2006AA020502”“06C26211200695”, Tianjin Grant “07JCZDJC03300” and “06ZHCXSH04800”.
Rights and permissions
About this article
Cite this article
Liu, Y., Yu, Y., Yang, S. et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother 58, 687–697 (2009). https://doi.org/10.1007/s00262-008-0591-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0591-5