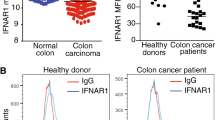
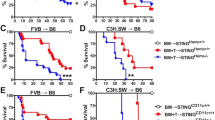
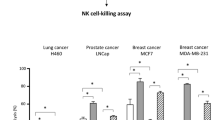
Abstract
Many tumors down-regulate major histocompatibility complex (MHC) antigen expression to evade host immune surveillance. However, there are very few in vivo models to study MHC antigen expression during tumor spontaneous regression. In addition, the roles of transforming growth factor beta1 (TGF-β1), interferon gamma (IFN-γ), and interleukin (IL)-6 in modulating MHC antigen expression are ill understood. We previously reported that tumor infiltrating lymphocyte (TIL)-derived IL-6 inhibits TGF-β1 and restores natural killing (NK) activity. Using an in vivo canine-transmissible venereal tumor (CTVT) tumor model, we presently assessed IL-6 and TGF-β involvement associated with the MHC antigen expression that is commonly suppressed in cancers. IL-6, IFN-γ, and TGF-β1, closely interacted with each other and modulated MHC antigen expression. In the presence of tumor-derived TGF-β1, host IFN-γ from TIL was not active and, therefore, there was low expression of MHC antigen during tumor progression. TGF-β1-neutralizing antibody restored IFN-γ-activated MHC antigen expression on tumor cells. The addition of exogenous IL-6 that has potent anti-TGF-β1 activity restored IFN-γ activity and promoted MHC antigen expression. IFN-γ and IL-6 in combination acted synergistically to enhance the expression of MHC antigen. Thus, the three cytokines, IL-6, TGF-β1, and IFN-γ, closely interacted to modulate the MHC antigen expression. Furthermore, transcription factors, including STAT-1, STAT-3, IRF-1, NF-κB, and CREB, were significantly elevated after IL-6 and IFN-γ treatment. We conclude that the host IL-6 derived from TIL works in combination with host IFN-γ to enhance MHC molecule expression formerly inhibited by TGF-β1, driving the tumor toward regression. It is suggested that the treatment of cancer cells that constitutively secrete TGF-β1 should incorporate anti-TGF-β activity. The findings in this in vivo tumor regression model have potential applications in cancer immunotherapy.










Similar content being viewed by others
References
Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL (1997) Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 18:89–95
Hoffmann TK, Meidenbauer N, Muller-Berghaus J, Storkus WJ, Whiteside TL (2001) Proinflammatory cytokines and CD40 ligand enhance cross-presentation and cross-priming capability of human dendritic cells internalizing apoptotic cancer cells. J Immunother 24:162–171
Overwijk WW (2005) Breaking tolerance in cancer immunotherapy: time to ACT. Curr Opin Immunol 17:187–194
Yu Z, Restifo NP (2002) Cancer vaccines: progress reveals new complexities. J Clin Invest 110:289–294
Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA (2006) Clonal origin and evolution of a transmissible cancer. Cell 126:477–487
Hsiao YW, Liao KW, Hung SW, Chu RM (2002) Effect of tumor infiltrating lymphocytes on the expression of MHC molecules in canine transmissible venereal tumor cells. Vet Immunol Immunopathol 87:19–27
Katzir N, Arman E, Cohen D, Givol D, Rechavi G (1987) Common origin of transmissible venereal tumors (TVT) in dogs. Oncogene 1:445–448
Liao KW, Lin ZY, Pao HN, Kam SY, Wang FI, Chu RM (2003) Identification of canine transmissible venereal tumor cells using in situ polymerase chain reaction and the stable sequence of the long interspersed nuclear element. J Vet Diagn Invest 15:399–406
Hsiao YW, Liao KW, Hung SW, Chu RM (2004) Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol 172:1508–1514
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146
Cross D, Cambier JC (1990) Transforming growth factor beta 1 has differential effects on B cell proliferation and activation antigen expression. J Immunol 144:432–439
Thomas DA, Massague J (2005) TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369–380
Panek RB, Lee YJ, Benveniste EN (1995) TGF-beta suppression of IFN-gamma-induced class II MHC gene expression does not involve inhibition of phosphorylation of JAK1, JAK2, or signal transducers and activators of transcription, or modification of IFN-gamma enhanced factor X expression. J Immunol 154:610–619
Ljunggren G, Anderson DJ (1998) Cytokine induced modulation of MHC class I and class II molecules on human cervical epithelial cells. J Reprod Immunol 38:123–138
Ting JP, Baldwin AS (1993) Regulation of MHC gene expression. Curr Opin Immunol 5:8–16
Wei L-H, Kuo M-L, Chen C-A, Chou C-H, Lai K-B, Lee C-N, Hsieh C-Y (2003) Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22:1517
Ohta K, Yamagami S, Taylor AW, Streilein JW (2000) IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 41:2591–2599
Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL (1993) Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-alpha/beta secretion. J Immunol 150:4844–4855
Scheid C, Young R, McDermott R, Fitzsimmons L, Scarffe JH, Stern PL (1994) Immune function of patients receiving recombinant human interleukin-6 (IL-6) in a phase I clinical study: induction of C-reactive protein and IgE and inhibition of natural killer and lymphokine-activated killer cell activity. Cancer Immunol Immunother 38:119–126
Cao X, Wang Q, Ju DW, Tao Q, Wang J (1999) Efficient inducation of local and systemic antitumor immune response by liposome-mediated intratumoral co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res 18:191–200
Liang CT, Chueh LL, Pang VF, Zhuo YX, Liang SC, Yu CK, Chiang H, Lee CC, Liu CH (2007) A non-biotin polymerized horseradish-peroxidase method for the immunohistochemical diagnosis of canine distemper. J Comp Pathol 136:57–64
Wagner AH, Gebauer M, Pollok-Kopp B, Hecker M (2002) Cytokine-inducible CD40 expression in human endothelial cells is mediated by interferon regulatory factor-1. Blood 99:520–525
Park YG, Nesterova M, Agrawal S, Cho-Chung YS (1999) Dual blockade of cyclic AMP response element- (CRE) and AP-1-directed transcription by CRE-transcription factor decoy oligonucleotide. gene-specific inhibition of tumor growth. J Biol Chem 274:1573–1580
Krzesz R, Wagner AH, Cattaruzza M, Hecker M (1999) Cytokine-inducible CD40 gene expression in vascular smooth muscle cells is mediated by nuclear factor kappaB and signal transducer and activation of transcription-1. FEBS Lett 453:191–196
Darnell JE, Kerr I, Stark G (1994) Jak-STAT pathways and transcriptional activation in response to interferons and other extracellular signaling proteins. Science 264:1415–1420
Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334(Pt 2):297–314
Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P (2001) DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem 276:6675–6688
Pazmany T, Tomasi TB (2006) The major histocompatibility complex class II transactivator is differentially regulated by interferon-gamma and transforming growth factor-beta in microglial cells. J Neuroimmunol 172:18–26
Ma D, Niederkorn JY (1995) Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 86:263–269
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693–699
McCartney-Francis NL, Wahl SM (2002) Dysregulation of IFN-gamma signaling pathways in the absence of TGF-beta 1. J Immunol 169:5941–5947
Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB (1993) Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci USA 90:9944–9948
Shouda T, Hiraoka K, Komiya S, Hamada T, Zenmyo M, Iwasaki H, Isayama T, Fukushima N, Nagata K, Yoshimura A (2006) Suppression of IL-6 production and proliferation by blocking STAT3 activation in malignant soft tissue tumor cells. Cancer Lett 231:176–184
Mule JJ, Custer MC, Travis WD, Rosenberg SA (1992) Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J Immunol 148:2622–2629
Usuda J, Okunaka T, Furukawa K, Tsuchida T, Kuroiwa Y, Ohe Y, Saijo N, Nishio K, Konaka C, Kato H (2001) Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer 93:475–480
Code S, Simard C, Lemieux R (2002) Regulation of growth-related genes by interleukin-6 in muring myeloma cells. Cytokine 20:113–120
Sakaguichi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352
Wang HY, Wang RF (2007) Regulatory T cells and cancer. Curr Opin Immunol 19:217–223
Zhang L, Yi H, Xia XP, Zhao Y (2006) Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity 39:269–276
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM (2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061
Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev 3:900–911
Balhoff JP, Stephens JM (1998) Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun 247:894–900
Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ (2001) Interferon-gamma modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology 142:3142–3151
Moreno CS, Beresford GW, Louis-Plence P, Morris AC, Boss JM (1999) CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity 10:143–151
van der Stoep N, Quinten E, van den Elsen PJ (2002) Transcriptional regulation of the MHC class II trans-activator (CIITA) promoter III: identification of a novel regulatory region in the 5’-untranslated region and an important role for cAMP-responsive element binding protein 1 and activating transcription factor-1 in CIITA-promoter III transcriptional activation in B lymphocytes. J Immunol 169:5061–5071
Huang S (2007) Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res 13:1362–1366
Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19:2474–2488
Kortylewski M, Kujawski M, Wang TH, Wei S, Zhang SM, Guilian STP, Kay NHK, William JM, Richard GK, Pardoll JW, Yu H (2005) Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 11:1314–1321
Aggarwal B, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H (2006) Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci 1091:151–169
Wagenka UM, Buschmann L, Lutticken C, Heinrich PC, Horn F (1993) Acute-phase response factor, a muclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol 13:2762–2788
Revel M, Katz A, Eisenbach L, Feldman M, Haran-Ghera N, Harroch S, Chebath J (1995) Interleukin-6: effects on tumor models in mice and on the cellular regulation of transcription factor IRF-1. Ann N Y Acad Sci 762:342–355; Discussion 355–346
Agresti C, Bernardo A, Del Russo N, Marziali G, Battistini A, Aloisi F, Levi G, Coccia EM (1998) Synergistic stimulation of MHC class I and IRF-1 gene expression by IFN-gamma and TNF-alpha in oligodendrocytes. Eur J Neurosci 10:2975–2983
Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD (1996) Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84:431–442
Chelbi-alix MK, Bobe P, Benoit G, Canova A, Pine R (2003) Arsenic enhances the activation of Stat1 by interferon gamma leading to synergistic expression of IRF-1. Oncogene 22:9121–9130
Wesoly J, Szweykowska-Kulinska Z, Bluyssen HA (2007) STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol 54:27–38
Kim OS, Park EJ, Joe EH, Jou I (2002) JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 277:40594–40601
Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ (1999) Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol 163:1428–1434
Acknowledgments
This project was supported by National Science Council of Taiwan (NSC95-2313-B-002-375) and Council of Agriculture of Taiwan (96AS-1.2.1-AD.U1(20)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsiao, YW., Liao, KW., Chung, TF. et al. Interactions of host IL-6 and IFN-γ and cancer-derived TGF-β1 on MHC molecule expression during tumor spontaneous regression. Cancer Immunol Immunother 57, 1091–1104 (2008). https://doi.org/10.1007/s00262-007-0446-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0446-5