Abstract
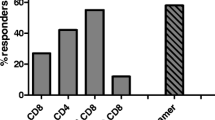
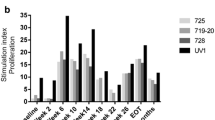
We have shown the immunogenicity and safety of synthetic carbohydrate vaccines when conjugated to the carrier keyhole limpet hemocyanin (KLH) and given with the adjuvant, QS-21, in patients with biochemically relapsed prostate cancer. To determine whether immune response could be further enhanced with stimulation by multiple antigens, a hexavalent vaccine was prepared using previously determined doses and administered in a Phase II setting to 30 high-risk patients. The hexavalent vaccine included GM2, Globo H, Lewisy, glycosylated MUC-1-32mer and Tn and TF in a clustered formation, conjugated to KLH and mixed with QS-21. Eight vaccinations were administered over 13 months. All 30 patients had significant elevations in antibody titers to at least two of the six antigens; 22 patients had increased reactivity with FACS. These serologic responses were lower than that seen previously in patients treated with the respective monovalent vaccines. The reciprocal median combined IgM and IgG antibody titers with ELISA against MUC1, Tn, TF, globo H and GM2 for these 30 patients were 640, 80, 120, 40 and 0, compared to 1280, 640, 1280, 320 and 160 seen in patients receiving individual monovalent vaccines. This hexavalent vaccine of synthetic “self” antigens broke immunologic tolerance against two or more antigens in all 30 vaccinated patients, was safe, but antibody titers against several of the antigens were lower than those seen in individual monovalent trials. No impact on PSA slope was detected. We address the relevance of the multivalent approach for prostate cancer treatment.





Similar content being viewed by others
References
Pound CR, Partin AW, Eisenberger MA et al (1999) Natural history of progression after PSA elevation following radical prostatectomy. J Am Med Assoc 281:1591–1597
Ragupathi R, Slovin SF, Adluri S et al (1999) A fully synthetic globo H carbohydrate vaccine induces a focused humoral response to prostate cancer patients: a proof of principle. Angew Chem Int Ed 38:563–566
Slovin SF, Ragupathi G, Musselli C et al (2003) Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine (Tn) conjugate vaccine. J Clin Oncol 21:4292–4298
Ragupathi G Koganty RR, Qiu DX et al (1998) A novel and efficient method for synthetic carbohydrate conjugate vaccine preparation: synthesis of sialyl Tn KLH conjugate using a 4-(4-N-maleimidomethyl)cyclohexane-1-carboxyl hydrazide (MMCCH) linker arm. Glycoconj J 15:217–221
Seeberger P, Cirillo PF, Hu S et al (1996) Synthesis of the pentasaccharide core structure of asparagine-linked glycoprotein oligosaccharides by the glycal assembly method. Enantiomer 1:311–323
Danishefsky SJ, Bilodeau MT (1996) Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew Chem Int Ed Engl 35:1380–1419
Helling F, Zhang S, Shang A et al (1995) GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res 55:2783–2788
Gilewski T, Adluri S, Zhang S et al (2000) Vaccination of high risk breast cancer patients with Mucin-1 keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res 6:1693–1701
Livingston P (1995) Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev 145:147–166
Slovin SF, Ragupathi G, Adluri S et al (1999) Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo hexasaccharide conjugate in man. Proc Natl Acad Sci USA 96:5710–5715
Slovin S, Ragupathi G, Clarke T et al (2003) Multivalency in a phase II prostate cancer (PC) vaccine trial: are more antigens better? Proc Am Soc Clin Oncol 22:167 (Abstr#671)
Kim SK, Ragupathi G, Musselli C, Livingston PO (1999) Comparison of the effect of different immunological adjuvants on the antibody and T cell response to immunization with MUC1-KLH and GD3-KLH conjugate vaccines. Vaccine 18:597–603
Tempero RM, VanLith L, Morikane K et al (1998) CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in viro and is absent in MUC1 transgenic mice. J Immunol 161:5500–5506
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: Protectin and control of the cell surface. Nat Rev 4:45–60
Agrawal B, Krantz MJ, Parker J, Longenecker BM (1998) Expression of MUC 1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res 58:4079–4081
Gimmi CD, Morrison BW, Mainprice BA et al (1996) Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human t cells. Nat Med 2:1367–1370
Wykes M, MacDonald KP, Tran M et al (2002) Quin RJ, Xing PX, Gendler SJ, et al. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J Leukoc Biol 72:692–701
Anderson P (1983) Antibody response to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of type b capsule with the nontoxic protein CRM197. Infect Immun 39:233–238
Kurika S (1996) Priming with diphtheria-tetanus-pertussis vaccine enhances the response to the Haemophilus influenzae type b tetanus conjugate vaccine in infancy. Vaccine 14:1239–1242
Barington T, Gyhrs A, Kristensen K, Heilmann C (1994) Opposite effect of actively and passively acquired immunity to the carrier on response of human infants to Haemophilus influenzae type b conjugate vaccine. Infect Immun 62:9–14
Peters CC, Tenbergen-Meeks AM, Poolman JT et al (1974) Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun 59:3504–3510
Sarvas H, Makela O, Toivanen P, Toivanen A (1974) Effect of carrier preimmunization on the anti-hapten response in chicken. Scand J Immunol 3:455–460
Fattom A, Cho YH, Chu C et al (1999) Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 17:126–133
Barington T, Skettrup M, Juul L, Heilman C (1993) Non-epitope-specific suppression of antibody response to Haemophilus influenzae type b conjugate vaccine by preimmunization with vaccine components. Infect Immun 61:432–438
Cleland JL, Kensil CR, Lim A et al (1996) Isomerization and formulation stability of the vaccine adjuvant QS-21. J Pharm Sci 85:22–28
Armstrong JL, Ragupathi G, Powell S et al (2003) Preliminary data of vaccination of high risk breast cancer (BC) patients with a heptavalent antigen: keyhole limpet hemocyanin (KLH) conjugate plus the immunologic adjuvant QS-21. Proc Am Soc Clin Oncol 675:168 (Abstr#675)
Slovin SF, Wilton A, Heller G, Scher HI (2005) Time to detectable metastatic disease in patients with a rising PSA after surgery or radiation therapy. Clin Cancer Res 11:8669–8673
Simons JW, Mikhak B, Chang JF et al (1999) Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res 59:5160–5168
Simons JW, Sacks N (2006) Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX® vaccine for prostate cancer. Urol Oncol 24(5):419–424
Livingston PO, Cunningham-Rundles S, Marfleet G et al (1987) Inhibition of suppressor-cell activity by cyclophosphamide in patients with malignant melanoma. J Biol Response Mod 6:392–403
Gerritsen W, Van den Eertwegh AJ, De Gruijl T et al (2006) A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (mHRPC). Proc Am Soc Clin Oncol 24:100 (Abstr#2500)
Scher HI, Eisenberger M, D’Amico AV et al (2004) Eligibility and outcomes, reporting guidelines for clinical trials for patients in the state of a rising PSA: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 22:537–56
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Prostate Cancer Foundation, The PepsiCo Foundation, The Sharon Hels and Brad Reed Fund, Swim Across America, The Sara Chait Foundation.
Dr. Philip Livingston is a consultant for and shareholder in Progenics Pharmaceuticals, Inc.
Rights and permissions
About this article
Cite this article
Slovin, S.F., Ragupathi, G., Fernandez, C. et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother 56, 1921–1930 (2007). https://doi.org/10.1007/s00262-007-0335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0335-y