Abstract
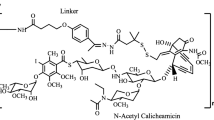
Antibody-targeted chemotherapy with immunoconjugates of calicheamicin is a clinically validated strategy in cancer therapy. This study describes the selection of a murine anti-CD22 mAb, m5/44, as a targeting agent, its conjugation to a derivative of calicheamicin (CalichDM) via either acid-labile or acid-stable linkers, the antitumor activity of CalichDM conjugated to m5/44, and its subsequent humanization by CDR grafting. Murine IgG1 mAb m5/44 was selected based on its subnanomolar affinity for CD22 and ability to be internalized into B cells. CalichDM conjugated to m5/44 caused potent growth inhibition of CD22+ human B-cell lymphomas (BCLs) in vitro. The conjugate of m5/44 with an acid-labile linker was more potent than an acid-stable conjugate, a nonbinding conjugate with a similar acid-labile linker, or unconjugated CalichDMH in inhibiting BCL growth. CalichDM conjugated to m5/44 caused regression of established BCL xenografts in nude mice. In contrast, both unconjugated m5/44 and a nonbinding conjugate were ineffective against these xenografts. Based on the potent antitumor activity of m5/44-CalichDM conjugates, m5/44 was humanized by CDR grafting to create g5/44, an IgG4 anti-CD22 antibody. Both m5/44 and g5/44 bound CD22 with subnanomolar affinity. Competitive blocking with previously characterized murine anti-CD22 mAbs suggested that g5/44 recognizes epitope A located within the first N-terminal Ig-like domain of human CD22. Antitumor efficacy of CalichDM conjugated to g5/44 against BCL xenografts was more potent than its murine counterpart. Based on these results, a calicheamicin conjugate of g5/44, CMC-544, was selected for further development as a targeted chemotherapeutic agent for the treatment of B-cell malignancies.








Similar content being viewed by others
Abbreviations
- AcBut:
-
4-(4′-Acetylphenoxy) butanoic acid
- AcPAc:
-
(3-Acetylphenyl) acetic acid
- ATC:
-
Antibody-targeted chemotherapy
- BCL:
-
B-cell lymphoma
- CalichDM:
-
N-Acetyl-γ-calicheamicin dimethyl disulfide derivative(s)
- CalichDMA:
-
CalichDM acid
- CalichDMH:
-
CalichDM hydrazide
- CDR:
-
Complementarity determining region
- NHL:
-
Non-Hodgkin’s lymphoma
- PBMC:
-
Peripheral blood mononuclear cell
- TAA:
-
Tumor-associated antigen
References
Trail P, Bianchi A (1999) Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol 11:584
Dubowchik G, Walker M (1999) Receptor-mediated and enzyme-dependent targeting of cytotoxic anti-cancer drugs. Pharmacol Ther 83:67
Damle NK, Frost P (2003) Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr Opin Pharmacol 3:386
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Willaims G, Pazdur R (2001) Gemtuzumab ozogamicin: approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 7:1490
Sievers E, Larson R, Stadmauer E, Estey E, Lowenberg BH, Dombret HC, Karanes C, Theobald M, Bennett JM, Sherman ML, Berger MA, Eten CB, Loken MR, van Dongen JJM, Bernstein ID, Appelbaum FR (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 19:3244
Larson R, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, Mineur P, Bennett JM, Berger MS, Eten CB, Muntean M, Loken MR, van Dongen JJM, Bernstein ID, Applebaum FR (2002) Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 16:1627
Berger M, Leopold L, Dowell J, Korth-Bradley J, Sherman M (2002) Licensure of gemtuzumab ozogamicin for the treatment of selected patients 60 years of age or older with acute myeloid leukemia in first relapse. Invest New Drugs 20:395
Hamann P, Hinman L, Beyer C, Lindh D, Upeslacis J, Flowers DA, Bernstein I (2002) An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia: choice of linker. Bioconjug Chem 3:40
Hamann P, Hinman L, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou HR, Upeslacis J, Schohat D, Mountain A, Flowers DA, Bernstein I (2002) Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem 13:47
Zein N, Sinha A, McGahren W, Ellestad G (1988) Calicheamicin γI: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science 240:1198
Lee MD, Dunne TS, Chang CC, Siegel MM, Morton GO, Ellestad GA, McGahren WJ, Borders DB (1992) Calicheamicins, a novel family of antibiotics, 4: Structural elucidations of calicheamicins. J Am Chem Soc 114:985
Thorson J, Sievers E, Ahlert J, Shepard E, Whitwam RE, Onwueme KC, Ruppen M (2000) Understanding and exploiting nature’s chemical arsenal: the past, present and future of calicheamicin research. Curr Pharm Des 6:1841
Mylotarg label. http://www.fda.gov/cder/foi/label/2000/21174lbl.pdf
Moyron-Quiroz JE, Partida-Sanchez S, Donis-Hernandez R, Sandoval-Montes C, Santos-Argumedo L (2002) Expression and function of CD22, a B-cell restricted molecule. Scand J Immunol 55:343
Tedder TF, Tuscano J, Sato S, Kehrl JH (1997) CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Ann Rev Immunol 15:481
Crocker PR, Varki A (2001) Siglecs, sialic acids and innate immunity. Trends Immunol 22:337
Nitschke L, Floyd H, Crocker PR (2001) New functions for the sialic acid-binding adhesion molecule CD22, a member of the growing family of Siglecs. Scand J Immunol 53:227
Hanna R, Ong GL, Mattes MJ (1996) Processing of antibodies bound to B-cell lymphomas and other hematological malignancies. Cancer Res 56:3062
Shan D, Press OW (1995) Constitutive endocytosis and degradation of CD22 by human B cells. J Immunol 154:4466
Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, Fitzgerald DJ, Pastan I (2001) Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 345:241
Pastan I, Kreitman RJ (2002) Immunotoxins in cancer therapy. Curr Opin Investig Drugs 3:1089
Hursey M, Newton DL, Hansen HJ, Ruby D, Goldenberg DM, Rybak SM (2002) Specifically targeting the CD22 receptor of human B-cell lymphomas with RNA damaging agents: a new generation of therapeutics. Leuk Lymphoma 43:953
Galfrè G, Howe SC, Milstein C (1977) Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature 266:550
Morgan A, Jones ND, Nesbitt AM, Chaplin L, Bodmer MW, Emtage JS (1995) The N-terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, Fc gamma RI and Fc gamma RIII binding. Immunology 86:319
Hinman LM, Hamann PR, Wallace R, Menendez AT, Dur FE, Upeslacis J (1993) Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res 53:3336
Coligan JE, Cruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) (1992) Current protocols in immunology, vol 2. Wiley, New York
Adair JR, Athwal DS, Emtage JS (1991) Humanised antibodies. International Patent Publication WO91/09967
Owens RJ, Young RJ (1994) The genetic engineering of monoclonal antibodies. J Immunol Methods 168:149
Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G (1992) The repertoire of human germline VH segments reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol 227:776
Cox JPL, Tomlinson IM, Winter G (1994) A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur J Immunol 24:827
Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C (1991) Sequences of proteins of immunological interest, 5th edn. Public Health Service, National Institutes of Health, Bethesda, MD
Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill J, Air G, Sheriff S, Padlan EA, Davies D, Tulip WR, Colman PM, Spinelli S, Alzari PM, Poljak RJ (1989) Conformations of immunological hypervariable regions. Nature 342:877
Li JL, Shen GL, Ghetie MA, May RD, Till M, Ghetie V, Uhr JW, Janossy G, Thorpe PE, Amlot P (1989) The epitope specificity and tissue reactivity of four murine monoclonal anti-CD22 antibodies. Cell Immunol 118:85
Engel P, Wagner N, Miller AS, Tedder TF (1995) Identification of the ligand-binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp Med 181:1581
Engel P, Wagner N, Smith H, Tedder TF (1995) Structure/function analysis of CD22: domains that mediate adhesion. In: Leukocyte typing V. Oxford University Press, Oxford, pp 526–527
Stein R, Belisle E, Hansen HJ, Goldenberg DM (1993) Epitope specificity of the anti-(B cell lymphoma) monoclonal antibody, LL2. Cancer Immunol Immunother 37:293
Griffiths GL, Govindan SV, Sharkey RM, Fisher DR, Goldenberg DM (2003) 90 Y-DOTA-hLL2: an agent for radioimmunotherapy of non-Hodgkin’s lymphoma. J Nucl Med 44:77
DiJoseph JF, Armellino DC, Boghaert E, Khandke K, Dougher Sridharan L, Kunz A, Hamann PR, Gorovits B, Udata C, Moran JK, PopplewelL AG, Stephens S, Frost P, Damle NK (2004) Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B lymphoid malignancies. Blood 103:1807
Hinman LM, Hamann PR, Upeslacis J (1995) Preparation of conjugates to monoclonal antibodies. In: Borders DB, Doyle TW (eds) Endiyne antibiotics as antitumor agents. Dekker, New York, pp 87–106
Co MS, Queen C (1991) Humanized antibodies for therapy. Nature 351:501
Burton DR, Woof JM (1992) Human antibody effector function. Adv Immunol 51:1
Greenwood J, Clark M, Waldmann H (1993) Structural motifs involved in human IgG antibody effector functions. Eur J Immunol 5:1098
Leonard JP, Coleman M, Ketas JC, Chadburn A, Ely S, Furman RR, Wegener WA, Hansen HJ, Ziccardi H, Eschenberg M, Gayko U, Cessano A, Goldenberg DM (2003) Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol 21:3051
Tuscano JM, O’Donnell RT, Miers LA, Kroger LA, Kukis DL, Lambom KA, Tedder TF, DeNardo GL (2003) Anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of 90 Y-DOTA-peptide-Lym-1 in lymphoma xenografts. Blood 101:3641
Acknowledgements
We would like to thank Dr John Crocker of Birmingham Heartlands Hospital, Birmingham, UK, for the evaluation of m5/44 binding to the NHL biopsies; Dr Lyka Kalyandrug for studies with effector functions of antibodies; Fred Immerman for statistical evaluation of results; and Maureen Dougher and Latha Sridharan for assistance with various studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DiJoseph, J.F., Popplewell, A., Tickle, S. et al. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol Immunother 54, 11–24 (2005). https://doi.org/10.1007/s00262-004-0572-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0572-2