Abstract
Purpose
To show the contribution of CEUS to characterization of indeterminate MRI observations in high-risk patients for hepatocellular carcinoma (HCC).
Methods
From July to December 2015, 42 consecutive patients referred to CEUS with indeterminate MRI scans comprise our study cohort. There are 50 indeterminate nodule-like observations and 10 arterial phase hyperenhancing foci, suggesting pseudolesions/arterio-portal shunts. MRI and CEUS lesions are classified according to their enhancement features in all phases and Liver Imaging and Reporting Data System (LI-RADS) in a blind read format. Clinical pathologic correlation and 24 months follow-up are performed.
Results
A majority, 37/50 (74%), of indeterminate nodule-like observations have arterial phase enhancement without washout on MRI. CEUS further characterizes enhancement and shows washout in 14/37 (38%). In total, CEUS diagnoses 16 malignant lesions in 14 patients including 14 HCC and 2 ICC. 12/16 (75%) malignant lesions are confirmed by biopsy or follow-up. Ultrasound identification of a nodule differentiates real nodules from pseudolesions. Of the ten suspected arterial-portal shunts on MRI, two show a real nodule on ultrasound, confirmed as an HCC and a regenerative nodule. 15/42 (36%) patients have LI-RADS escalated from LR-3 or 4 on MRI to LR-4 or 5 on CEUS. Overall, the sensitivity of CEUS is (13/16) 81.3% and specificity is (37/37) 100% for malignant diagnosis.
Conclusion
Grayscale ultrasound detects true nodules. Dynamic CEUS detects and characterizes washout, correctly predicting HCC. CEUS is complimentary to MRI and can serve as a problem-solving tool when MRI is indeterminate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), the most common primary liver malignancy, evolves by stepwise progression from a benign regenerative nodule (RN) into a dysplastic nodule (DN) prior to the development of well, moderately and poorly differentiated HCC [1,2,3]. The vascular changes which occur with the progression of cirrhotic nodules ultimately give the appearance of arterial phase (AP) hyperenhancement (APHE) and washout (WO) with contrast administration.
MRI is the mainstay for liver imaging in most tertiary institutions in North America. On MRI with gadolinium, typical HCC shows APHE and portal venous (PVP) or delayed phase (DP) WO, with signal changes on T1, T2, and diffusion weighted imaging (DWI) [4,5,6]. Stringent criteria for HCC have been developed by ACR LI-RADS®v2018 as LI-RADS (LR)-5 [7]. Lesions which do not meet these criteria are considered indeterminate [8]. This most often shows as APHE without WO [9]. The presence of WO is essential for diagnosis of HCC on MRI and CT [8, 10, 11], as many MRI observations that do not demonstrate WO may represent arterio-portal shunting (APS) [9]. Currently, according to the practice guidelines of the major international liver organizations, CT and MRI are the main techniques for evaluation of HCC with resolution of indeterminate observations by evaluation with the alternate modality, follow-up imaging, or biopsy, with no preference listed [8]. Recently updated AASLD 2018 guidelines also recommend CEUS as a troubleshooting tool in further characterization of the indeterminate lesions in expert centers [7].
CEUS is an emerging technique with unique diagnostic capabilities [12,13,14] of real-time monitoring of vascular enhancement compared to images acquired at static fixed time points on CT and MRI. Ultrasound is most sensitive to its contrast agent, allowing for superior detection of APHE and WO [12]. In addition, using purely intravascular microbubble contrast agents, CEUS offers precise resolution of WO [15, 16], eliminating the problem of pseudo-enhancement shown on CT and MRI related to interstitial leak of contrast agent [13]. In our institution, CEUS is well integrated in our multi-modality approach to diagnosis and management of HCC [14], often, providing non-invasive resolution of indeterminate MRI.
We hypothesize that the addition of CEUS in the further characterization of indeterminate MRI observations in high-risk patients for HCC will provide rapid non-invasive resolution of indeterminate MRI observations with additional diagnosis, including malignant and benign tumors, and reduce the time interval between the indeterminate MRI scan to the final diagnosis and treatment.
Materials and methods
Study population
This prospective study is approved by the Institutional Research Ethics Board. All patients provided signed informed consent. Between July and December 2015, 42 consecutive patients with indeterminate MRI results were referred to our tertiary ultrasound facility for liver CEUS as part of standard care, where they were prospectively recruited for our study.
Inclusion criteria
All patients are at high risk for developing HCC [7, 8]. All have MRI scans with indeterminate results. Most often are observations that do not meet the criteria for LR-5. Included are patients on primary surveillance (patients without previous HCC diagnosis or treatment, n = 18 patients) and on secondary surveillance (patients with previously treated HCC) who presented with new isolated nodules, unrelated to their original treatment (n = 24 patients). CEUS and MRI were performed within a 3 month time period with a median time interval of 25 days.
Exclusion criteria
Patients with definitive MRI results, CEUS performed outside of 3 months from the date of MRI, and allergy to CEUS contrast agent were excluded.
Our study cohort includes two groups. Group 1: 38 patients with 50 nodule-like observations on MRI with signal abnormalities on T1, T2, and DWI that suggest a real nodule. Group 2: 7 patients with 10 arterial-hyperenhancing foci without signal abnormality on T1, T2, or DWI, suggestive of APS. There are a number of patients who have observations in both groups (Supplemental Table 1). The study cohort is 64 years old on average, consists of 30/42 (71%) males. Risk factors include 41/42 (98%) cirrhosis due to 7/42 (17%) HBV, 25/42 (60%) HCV, 10/42 (24%) ETOH, and 4/42 (10%) NAFLD, and (1/42, 2%) chronic HBV without cirrhosis (Table 1) (the number of patients with each risk factor adds to more than 42 as 5 patients each have 2 risk factors).
Lesion diagnosis and follow-up
Lesions are classified based on their imaging characteristics as pseudolesions/APS, benign (RN, DN), and malignant nodules (HCC, Intrahepatic cholangiocarcinoma (ICC)). Reference standard includes biopsy (n = 9, 7 diagnostic and 2 non-diagnostic) and follow-up with imaging for interval stability. All patients, as possible, are followed for 2 years. At our institution, typical LR-5 lesions on CEUS or MRI are often treated without biopsy [7, 8, 14,15,16]. In 6 patients, ablative therapy was performed for CEUS LI-RADS 5 lesions without biopsy. In 2/6, subsequent development of a recurrent tumor at the ablation site confirmed a malignant diagnosis. Nodules are considered benign when they remain stable for a minimum of 24 months. Interval growth is defined as more than a 30% diameter change in 6 months. 2 patients are lost to follow-up: one deceased and the other with liver transplant. The rest of the cohort have follow-up for 24 months, often with alternating CEUS and MRI at 3-month intervals.
Procedures/techniques
MRI
Standard institutional MRI is performed at four tertiary facilities within our university, with gadovist (N = 39) or hepatobiliary contrast, gadoxetate disodium (N = 3). AP is taken at 30 s, PVP is taken at 70 s, and DP is taken at 120 s for extracellular contrast gadobutrol (Bayer, Canada) and 20 min for gadoxetate disodium (Bayer, Canada). The indeterminate MRI observations were blindly reviewed by two radiologists, one with 11-year subspecialty experience in liver imaging and the first author of this manuscript, a radiology resident in liver imaging. Consensus opinion was used in this analysis following deliberation of the two readers. Resolution of conflict in interpretation is performed by a third reader with 5 years of liver MRI experience. Signal intensity of lesions is compared to liver, as hypointense, isointense, or hyperintense, on T1, T2, and DWI sequences. T2 signal is assessed on a T2 TSE fat sat sequence (T2 FS, TR 4924 ms, TE 116 ms). T1 signal is assessed on T1 GRE in/out of phase sequence (T1 GRE, TR 122 ms, TE 2.37 and 4.78 ms). DWI signal is assessed at two b-values (b 100 and b 250). Intensity of contrast enhancement in all phases is classified as isointense, hyperintense, and hypointense on the basis of its brightness relative to the adjacent liver. AP enhancement patterns include non-rim APHE, rim APHE, non-peripheral WO, peripheral WO, and an enhancing capsule [7, 8, 17]. Observations were categorized according to CT/MRI LI-RADS®v2018 [7].
Ultrasound and CEUS
Ultrasound and CEUS scans are performed in a single tertiary facility with a high level of professional and technical expertise in all staff and technologists. Grayscale scan of the liver confirms whether there is a real underlying nodule. For patients with a real nodule, following complete grayscale scan of the liver, CEUS is performed according to our established protocols [14] on commercially available ultrasound equipment, utilizing a contrast-specific mode with a low mechanical index (0.06–0.2) to achieve stable and non-destructive microbubble imaging. Time 0 corresponds with initiation of the saline flush. Subsequent images of the lesion are obtained every 30 s to assess WO up to 300 s throughout the PVP (from 30 s to 2 min) and late phase (LP) (2–5 min). Perflutren lipid microspheres (Lantheus Medical Imaging, Billerica, MA, USA) are injected intravenously in a bolus of 0.2 mL to a maximum dose of 10 uL/kg followed immediately by a 5 mL saline flush, repeated up to five times as needed. The pattern of APHE is recorded continuously, from the arrival of the first bubble in the field of view, to the peak of AP enhancement of the lesion or the liver, and briefly beyond. Intensity is classified as hyperenhancement, isoenhancement, and hypoenhancement on the basis of its brightness relative to the adjacent liver. The enhancement patterns include non-rim diffuse APHE, referred to as APHE; rim APHE; and hemangioma-like APHE. Subsequently, WO is characterized based on its timing (early < 60 s and late WO > 60 s) and intensity (weak or marked, based on its first observation to 2 min). Nodules are categorized according to CEUS LI-RADS®v2017 [15, 16].
When no definite nodule is seen on grayscale ultrasound, CEUS may be performed focused on either an indeterminate observation or on a focus of APHE from prior MRI, utilizing anatomic landmarks. If there is APHE or WO seen on the CEUS examination, closer scrutiny of the region on grayscale ultrasound may reveal a subtle nodule. Additionally, if only washout is shown on CEUS corresponding to the original observation, CEUS may be performed “on top” by injecting the contrast agent while looking at the washout site. Therefore this improves nodule detection and characterization of the nodule. This described technique is not recommended in basic CEUS LI-RADS®v2017 [15, 16] and is advised for advanced users. The associated CEUS imaging files for the indeterminate MRI studies were blindly reviewed by two different radiologists, one with over 15 years of CEUS experience, and the other with 2 years of experience. Resolution of conflict in interpretation was performed by a third reader with 6 years of liver CEUS experience. Consensus opinion was used in all analysis.
CEUS and MRI were interpreted blindly and without any association to the other modality. The time interval between the interpretation and blind review limits recall bias, and the reviewers had no role in selection of the images for review.
Time to diagnosis is calculated from the time of an indeterminate MRI to the time a diagnosis is given.
Statistical analysis
The statistical analysis is performed using R program (3.5.0, Vienna, Austria). Diagnostic accuracy measures of CEUS, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), are calculated on the lesions that had confirmation using a reference standard. The lesions that had indeterminate CEUS diagnosis (N = 1), and lesions that do not have reference standard confirmation are not included in the analysis.
Results
Indeterminate MRI observations most often show APHE with no WO on MRI
Of the 50 nodule-like observations within group 1, 40/50 (80%) show APHE. The majority, 37/50 (74%) show APHE without WO on MRI (Table 2), including 16/37 (43%) showing APHE only without altered signal in T1, T2, or DWI. Of the 10 suspect APS within group 2, 10/10 (100%) observations show APHE only without WO or altered signal in T1, T2, and DWI.
Provision of a diagnosis on CEUS of indeterminate MRI observations
The diagnosis and the outcome of the indeterminate MRI cohort are shown in Fig. 1. From group 1, within the 28 observations showing a nodule on ultrasound, CEUS diagnoses 16/28 (57%) malignant nodules in 14 patients, including 14 HCC in 12 patients and 2 ICC in 2 patients. 12/16 (75%) are confirmed with reference standard, while the rest are treated immediately without biopsy. 11/28 (39%) nodules in 9 patients are shown to be benign, including 5 DN and 4 RN that are stable on follow-up. 1/28 (4%) nodules in 1 patient remains indeterminate after the CEUS evaluation. From group 2, suspected APS on MRI (n = 10), 2/10 show a nodule on ultrasound, one diagnosed as HCC (Fig. 4) and one as RN on CEUS, both are confirmed on follow-up. 3 nodules were missed on CEUS and considered as APS, but shown to be malignant on follow-up MRI.
The study cohort. In a 6-month interval, 42 consecutive patients with indeterminate MRI were referred for CEUS. White boxes indicate the basis for the indeterminate result on MRI. Yellow boxes are CEUS results and green boxes are reference standard confirmation including biopsy and follow-up. Pts patients, APS arterio-portal shunts, Path pathology, f/u follow-up, Tx treated, w/o without. Final (final diagnosis)
Overall, from 54/60 (90%) nodules with reference standard confirmation, the sensitivity of CEUS in detecting malignant lesions from indeterminate MRI observations is 81.3% (13/16; 95% confidence interval (CI): 54%, 96%) and specificity is 100% (37/37; 95% CI: 91%, 100%). PPV is 100% (13/13; 95% CI: 75%, 100%) and NPV is 93% (38/41; 95% CI: 80%, 98%). Number of patients in two groups adds to 45 as 3 patients have nodules in both groups.
Median time from identification of an indeterminate MRI observation to diagnosis is 33 days with addition of CEUS. Average time is 17.8 days.
Addition of grayscale ultrasound differentiates pseudolesions/APS from true nodules
As shown in Fig. 1: of 50 nodule-like observations on MRI in group 1, 28/50 (56%) are confirmed on grayscale ultrasound as real nodules. Sizes range from 1 to 4.5 cm with a median of 2.2 cm (Supplemental Table 2). The remaining 22/50 (44%) that show no nodule on grayscale ultrasound are regarded as pseudolesions/APS. Of the 10 suspect APS on MRI in group 2, CEUS shows 2 true nodules on grayscale ultrasound. The remaining 8 are totally negative on ultrasound/CEUS and are stable or disappear on follow-up MRI for 2 years, supportive of the suspicion of APS.
The addition of CEUS further assists with identification of indistinctive nodules on greyscale ultrasound. From 28 identified nodules, only 25 nodules were initially identified on greyscale ultrasound. Three more were identified when CEUS showed arterial enhancement features, localized to a subtle nodule on ultrasound. The other three nodules were missed on greyscale ultrasound and CEUS and are described in the section under false negative results. Thus, the sensitivity of using the greyscale ultrasound to identify a nodule is 25/31 (81%) and with CEUS is 28/31 (90%).
CEUS diagnosis of malignant nodules by demonstrating WO that is not shown on MRI
From the majority MRI observations from group 1 that show APHE with no WO, subsequent CEUS shows APHE with WO in 12/37 (32.4%). WO is weak in 10/12 HCC (Fig. 2, Supplemental Figs. 2 and 4, Table 3) and marked in 2/12 ICC (Fig. 3; Table 3). From group 2, suspect APS without WO on MRI, one nodule shows weak WO, diagnosed as an HCC (Fig. 4).
79-year-old male with ETOH cirrhosis on primary surveillance with an indetermiante nodule-like observation on MRI, diagnosed as an HCC on CEUS. a Diffusion weighted imaging (DWI) shows a 1.9 cm observation on the surface of segment VI (arrow). (Supplemental Fig. 2) It is isointense on T1 and T2. b On post-gadolinium imaging, there is homogeneous arterial phase (AP) hyperenhancement (APHE). c Delayed phase (DP) image shows persisting enhancement with no WO, LR-3, upgraded to LR-4 with ancillary features of positive DWI on MRI. d On greyscale ultrasound, a corresponding hypoechoic nodule shows (e) diffuse APHE on CEUS with (f) weak and late WO in late phase (LP), classic appearance of HCC on CEUS, LR-5. Biopsy confirmed well-differentiated HCC
61-year-old female with ETOH and HCV cirrhosis on secondary surveillance with an indeterminate nodule-like observation on MRI, diagnosed as ICC on CEUS. (Supplemental Fig. 3a–e) On MRI, this observation is isointense on T1 weighted image and has heterogeneous T2 and DWI signal. Post-gadolinium images show a solitary 2.4 cm observation with a heterogeneous APHE and (supplemental Fig. 3f) no WO on DP, LR-4 on MRI. b Greyscale ultrasound shows a heterogeneous nodule. c CEUS shows rapid enhancement at 10 s, and d punched-out WO at 23 s. This suggests LR-M, a probable malignant lesion without specificity for HCC, prompting biopsy, showing ICC
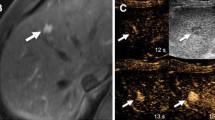
59-year-old female with HCV cirrhosis on primary surveillance who has an APS on MRI, diagnosed as HCC on CEUS. (Supplemental Fig. 3a–e) A suspect APS of 1.0 cm in size is isointense on T1, T2 and DWI. Post-gadolinium MRI image shows a hyperenhancement on AP and (Supplemental Fig. 3f) isointense on DP with no WO (LR-3). b On greyscale ultrasound, a hypoechoic nodule corresponds with the MRI focus of APHE. c CEUS shows diffuse APHE at 19 s and d weak WO at 3 min in LP, a classic HCC appearance (LR-5)
Additionally, from group 1, CEUS is highly suspicious for 2 HCC by showing a nodule on grayscale ultrasound and APHE with no WO (LR-4), which are confirmed as HCC on follow-up scan. Identification of APHE with a grayscale nodule is associated with a CEUS diagnosis of HCC in 17 nodules from both groups, equal to HCC diagnosis using both APHE with WO.
CEUS diagnosis of benign lesions which do not show APHE or WO
From group 1, indeterminate nodule-like observations, CEUS, diagnosed 9 benign lesions, including 5 DN and 4 RN (Supplemental Table 3). RN and DN show AP iso or hypoenhancement with no WO and are categorized as LR-3. From group 2, the suspected APS on MRI, CEUS diagnosed 1 RN, which shows isoenhancement on both arterial and late phase. None of these lesions are malignant on follow-up.
False negative results on initial CEUS evaluation of indeterminate MRI
From group 1, with a nodule-like observation on MRI, 22/50 patients show no nodule on initial ultrasound, 19/22 show interval stability on follow-up. However, 3/22 patients are ultimately diagnosed with HCC. One 1.1 cm lesion is occult on two CEUS exams separated by 6 months apart. An additional 1 cm nodule is shown to be HCC on both MRI and CEUS at 6 months follow-up and was likely missed on initial CEUS. The last 1 cm lesion was not detected on a poor CEUS scan.
CEUS as an alternate modality when MRI fails or is contraindicated
Five patients are not successfully evaluated on MRI due to severe respiratory motion (n = 2), AP phase timing error (n = 1), renal failure (n = 1), and failure of MRI to confirm the nodule identified on surveillance ultrasound (n = 1) (Table 2). From this small population, there is one HCC on CEUS with typical APHE and WO (Table 3) and four DNs in 4 patients.
Discussion
MRI is the primary imaging modality for assessment of cirrhotic nodules chosen by our tertiary hepatobiliary multidisciplinary team. However, for over a decade, CEUS has been well integrated into our multi-modality imaging methods [14]. This prospective study investigates the contribution of CEUS to the evaluation of consecutive indeterminate observations on MRI in high-risk patients collected in a 6-month interval, including 24 months follow-up. Although APHE with no WO accounts for the majority of the indeterminate MRI cases, there are a myriad of enhancement patterns on CEUS suggesting pseudolesions, benign lesions, and malignant lesions, including 14 HCC and 2 ICC. These results significantly impact patients’ treatment and prognosis and reduce the time to diagnosis. In the USA, where a microbubble contrast agent for liver imaging is approved only within the last 3 years and CEUS LI-RADS®v2017 is now published on the ACR website, we hope this information will influence utilization of CEUS in liver facilities in the future.
The significance of indeterminate MRI observations is previously described in a small number of retrospective studies [6, 9, 17,18,19,20], where the diagnosis of HCC is made by biopsy or interval growth on MRI. In our study, CEUS reaches the sensitivity of 81.3% and specificity of 100% in diagnosing malignant lesions from the indeterminate MRI observations. We confirm malignancy in 22% of indeterminate MRI observations. This rate of malignancy is comparable with two other studies citing 14% [18] and 21% [20] respectively.
This incidence of HCC in indeterminate nodule-like observations on MRI suggests further characterization and early intervention is beneficial [18, 20]. Adding CEUS to the current diagnostic algorithm provides earlier diagnosis. One study documenting sequential MRI examinations shows that the time from identification of an indeterminate observation to diagnosis of HCC is 6–24 months, with 73% of HCC having prior MRI suggesting benign or indeterminate observations [6]. In our study, with integration of CEUS into a multi-modality approach, the median time from identification of indeterminate observations on MRI to diagnosis of malignant lesions on CEUS is 33 days, average 17.8 days. This is significantly shorter than the time reported in the literature, where CEUS is not used [7, 20, 21].
Consistent with prior studies [9], lack of WO on MRI is the main reason for indeterminate MRI result in our study. As CEUS is real-time imaging performed with a purely intravascular microbubble contrast agent, it is very sensitive at detecting WO, thus identifying malignant lesions from indeterminate MRI observations. In addition, CEUS can discern the timing and intensity of the WO, which facilitates the differentiation of ICC from HCC [15, 16]. This is supported by the biopsy results in this study and other studies in our institution [15].
Ultrasound also plays an important role in differentiating real lesions from APS. Our study shows the sensitivity of ultrasound to detect a nodule from the indeterminate MRI observations is 81%, and with CEUS increases to 90%. CEUS makes the indistinguishable or subtle nodule on greyscale ultrasound more easily detected; facilitating CEUS guided biopsy and local ablative therapy. Additionally, this study supports previous evidence that AP hyperenhancement with an associated nodule on CEUS has high specificity for diagnosis of HCC [22].
This study describes the use of the contrast agent Definity (perflutren microspheres, Lantheus Medical Imaging, Billerica MA), which is approved by our health authority for liver mass characterization since 2002. We do not regard this as problematic for the American ultrasound scene, where the approved microbubble agent today is Sonovue (Sulfur hexafluoride) (Bracco, Milan). As these agents are from the same generation, they are clinically indistinguishable [23]. They are both used today in our facility without obvious differentiation.
Strengths of our study include its prospective collection of consecutive cases with indeterminate MRI observations within a 6 month time frame. In addition, we are a tertiary center, in which CEUS has been fully integrated within a multi-modality discipline for over a decade, for the diagnosis of liver nodules in patients at risk for HCC. This experience gave us the insight to evaluate CEUS for problem solving.
Our study has its limitations. It is performed in a single center where CEUS is well established. This may make generalization of our results to other less experienced departments more difficult.
Patients in this study are recruited through our CEUS clinic, thus the indeterminate MRIs may not represent the full spectrums of MRIs that are performed. However, our patients’ characteristics are very similar to other indeterminate MRI studies and are similar to what the new AASLD guidelines describe, with HCV being the primary risk factor for HCC [7, 8]. Additionally, the fact that many of the patients have indeterminate MRI performed before CEUS may provide ultrasound an advantage for finding pathology. However, all CEUS examinations of the liver are performed as total studies in our institution as all patients have a full greyscale evaluation with selection of all suspicious nodules for the performance of CEUS. Further, from our clinical experience, CEUS maybe performed prior to MRI without removing the likelihood of MRI indeterminate result. Time interval between CEUS and MRI is limited to 3 months, median time interval of 25 days, to reduce the possible impact of tumor evolution on the validity of the study.
Our study did not exclude the patients from secondary surveillance, where an indeterminate nodule is more likely to be malignant. However, primary mixed with secondary surveillance reflects the actual practice of imaging in all hepatobiliary centers. Regardless, CEUS identifies a range of cirrhotic nodules, both benign and malignant, in patients with and without prior HCC.
The biopsy rate in our study is low at < 30%, as some CEUS LR-5 lesions were treated without biopsy. This is consistent with current AASLD guidelines, recommending against biopsy due to its invasiveness, risk of seeding the tumor along the biopsy track, and a 30% false negative rate. The LI-RADS®v2018 also suggests LR-5 lesions on CEUS and MRI can be treated without biopsy.
Regarding the accuracy of CEUS in diagnosing indeterminate MRI observations, the very high specificity of CEUS is partially explained by the preselection of the population with indeterminate MRI observations, where the majority of malignant lesions are excluded. Further, inter- and intra-observer variability was not performed.
Performance of the study has made us aware that both MRI and ultrasound have occult nodules on baseline imaging which show only their enhancement patterns on contrast studies. This will be addressed in future investigations.
Conclusion
MRI is the mainstay for liver cancer imaging,; however, indeterminate MRI results are common. CEUS provides non-invasive resolution of indeterminate MRI observations and reveals new information for tumor diagnosis. In addition to superior detection of real nodules and differentiation of real nodules from pseudolesions, CEUS better detects DP washout when MRI shows only APHE, correctly predicting malignant HCC and ICC. CEUS is a good problem-solving tool. It complements MRI and expedites diagnosis and treatment of liver lesions.
References
International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995; 22:983–993.
Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991; 22:172–178.
Matsui O, Gabata T, Kobayashi S, et al. Imaging of multistep human hepatocarcinogenesis. Hepatol Res. 2007; 37 Suppl 2:S200–S205
Digumarthy SR, Sahani DV, Saini S. MRI in detection of hepatocellular carcinoma (HCC), Cancer Imaging. 2005; 13(5): 20-24
Hayashi M, Matsui O, Ueda K, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR 1999; 172:969–976.
Choi D, Mitchell DG, Verma SK, et al. Hepatocellular carcinoma with indeterminate or false-negative findings at initial MR imaging: effect on eligibility for curative treatment—initial observations, Radiology: 2007; 244 (3):776-83
ACR LIRADS®v 2018, https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67 (1): 358-380.
Choi JY, Cho HC, Sun M, et al. Indeterminate observations (liver imaging reporting and data system category 3) on MRI in the cirrhotic liver: fate and clinical implications. AJR 2013; 201(5):993-1001
Shimizu A, Ito K, Koike S, et al. Cirrhosis or chronic hepatitis: evaluation of small (< or = 2-cm) early enhancing hepatic lesions with serial contrast-enhanced dynamic MR imaging. Radiology. 2003; 226:550–555.
Holland AE, Hecht EM, Hahn WY, et al. Importance of small (< or = 20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology. 2005; 237: 938–944.
Sugimoto K, Moriyasu F, Shiraishi J, et al. Assessment of arterial hypervascularity of hepatocellular carcinoma: comparison of contrast-enhanced US and gadoetate disodium-enhanced MR imaging. Eur J Radiol. 2012; 22(6):1205-1213.
Wilson SR, Kim TK, Jang HJ, et al. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR 2007; 189:W7-W12.
Jo PC, Jang HJ, Burns PN, et al. Integration of contrast-enhanced ultrasound into a multimodality approach to imaging of nodules in a cirrhotic liver: How I do it. Radiology. 2017; 282(2):317-331.
Wilson SR, Lyshchik A, Piscaglia F, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol. 2018; 43(1):127-142.
Kono Y, Lyshchik A, Cosgrove D, et al. Contrast enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS): the official version by the American College of Radiology (ACR), 2017.
Elsayes KM, Hokker JC, Agrons MM, et al. 2017 Version of LI-RADS for CT and MR imaging: an update. Radiographics. 2017; 37(7): 1994-2017.
Khalili K, Kim TK, Jang HJ, et al. Indeterminate 1-2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011;54 (6):2048-54
Quaia E. Solid focal liver lesions indeterminate by contrast-enhanced CT or MR imaging: the added diagnostic value of contrast-enhanced ultrasound. Abdom Imaging. 2012; 37(4):580-90.
Beal EW, Albert S, McNally M, et al. An indeterminate nodule in the cirrhotic liver discovered by surveillance imaging is a prelude to malignancy. J Surg Oncol. 2014;110(8):967-9
Beal EW, Kearney JF, Chakedis JM, et al. Interval magnetic resonance imaging: an alternative to guidelines for indeterminate nodules discovered in the cirrhotic liver. J Gastrointest Surg. 2017; 21(9):1463-1470.
Jang HJ, Kim TK, Burns PN, et al. Enhancement pattern of hepatocellular carcinoma at contrast enhanced ultrasound: comparison with histologic differentiation. Radiology. 2007; 244 (3): 898-906.
Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2015, 24 (1): 3-18.
Acknowledgement
The authors thank Glen Pridham, MSc for assistance with statistics analysis.
Funding
This research project did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hu, J., Bhayana, D., Burak, K.W. et al. Resolution of indeterminate MRI with CEUS in patients at high risk for hepatocellular carcinoma. Abdom Radiol 45, 123–133 (2020). https://doi.org/10.1007/s00261-019-02181-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02181-2