Abstract
Purpose
This study aims to investigate the role of diffusion-weighted imaging (DWI) and T2-weighted imaging (T2WI) in combination for the detection of prostate cancer, specifically assessing the role of high b-values (> 1000 s/mm2), with a systematic review and meta-analysis of the existing published data.
Methods
The electronic databases MEDLINE, EMBASE, and OpenSIGLE were searched between inception and September 1, 2017. Eligible studies were those that reported the sensitivity and specificity of DWI and T2WI for the diagnosis of prostate cancer by visual assessment using a histopathologic reference standard. The QUADAS-2 critical appraisal tool was used to assess the quality of included studies. A meta-analysis with pooling of sensitivity, specificity, likelihood, and diagnostic odds ratios was undertaken, and a summary receiver-operating characteristics (sROC) curve was constructed. Predetermined subgroup analysis was also performed.
Results
Thirty-three studies were included in the final analysis, evaluating 2949 patients. The pooled sensitivity and specificity were 0.69 (95% CI 0.68–0.69) and 0.84 (95% CI 0.83–0.85), respectively, and the sROC AUC was 0.84 (95% CI 0.81–0.87). Subgroup analysis showed significantly better sensitivity with high b-values (> 1000 s/mm2). There was high statistical heterogeneity between studies.
Conclusion
The diagnostic accuracy of combined DWI and T2WI is good with high b-values (> 1000 s/mm2) seeming to improve overall sensitivity while maintaining specificity. However, further large-scale studies specifically looking at b-value choice are required before a categorical recommendation can be made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With a crude incidence of 134.3 per 100,000, prostate cancer is the most common cancer in men, and the second-biggest cause of cancer mortality [1, 2]. The quoted incidence has increased in recent years; however, this may be due to the use of prostate-specific antigen (PSA) blood testing. The majority of suspected cases with either a high PSA, abnormal digital rectal examination (DRE), or suggestive symptoms, will undergo a transrectal ultrasound guided biopsy (TRUS) to confirm and grade a histopathologic diagnosis [3]. If this is positive and the patient is a candidate for radical treatment, they will receive multiparametric magnetic resonance imaging (mpMRI) to assess the extent of cancer growth. However, there are now a substantial number of centers choosing pre-biopsy mpMRI followed by a more-targeted biopsy.
Multiparametric MRI is a well-established imaging modality for assessing prostate cancer, predominately to exclude extra-glandular spread and to judge how much of the prostate is involved. It consists of multiple sequences, including T1- and T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) and, in some instances, Dynamic contrast-enhanced (DCE) imaging. Multiple meta-analyses have proven DWI to have good diagnostic accuracy [4,5,6]; its contrast is governed by numerous technical parameters, one of the most important of these is the diffusion-weighting factor, or ‘b-value’. The b-value reflects the strength and timings of magnetic field gradients applied to the patient, and acquisition of multiple b-values permits calculation of an apparent diffusion coefficient (ADC) map, which gives a quantitative measure of tissue diffusion that has been shown to have an inverse correlation with tumor Gleason score [7]. Currently the recommendation is to use at least two b-values, one of 50–100 s/mm2, 800–1000 s/mm2 and if possible 1400–2000 s/mm2 [8, 9]. Theoretically, increasing the maximum b-value results in a better contrast-to-noise ratio (CNR) because there is greater suppression of normal prostate tissue signal, so resulting tumors are more apparent. However, the tradeoff is a reduced signal-to-noise ratio (SNR). Even though b-values > 1400 is recommended, there is little evidence supporting this and there is no widely accepted optimal “high b-value.” In a previous meta-analysis, Wu et al. showed no benefit from increasing b-value but only one paper in the analysis used b-values of over 1000 [4]. A multitude of recent studies have shown high sensitivity and specificity with higher b-values using both visual and ADC value assessments [10,11,12]. For clinical relevance, we hope to investigate the diagnostic accuracy achievable by visual assessment of DWI in combination with T2WI at high b-values > 1000 s/mm2.
Materials and methods
This review was registered with the PROSPERO International prospective register of systematic reviews (reference number: 42016036196) prior to commencement [13]. The review was carried out in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidance [14].
A systematic review of the literature was independently undertaken by two reviewers, who identified studies that investigated the diagnostic accuracy of DWI and T2WI MRI in the detection of prostate cancer. Searches were performed using MEDLINE and EMBASE electronic databases, as well as OpenSIGLE to explore sources of unpublished gray literature. The Science Citation Index was used to identify articles which cite those identified with the original search terms. Once eligible studies were found, their reference lists were manually searched for further potential papers. The search strategy for MEDLINE, including Boolean operators and MeSH terms, is presented in Table 1; the same search strategy was used for each database with alterations to suit. All studies were included up to the date of the search: 1st of September 2017.
Eligibility
The eligibility criteria for the studies included within the systematic review were that they used both DWI and T2WI MRI in combination for the assessment of prostate cancer; they were applied for the assessment of the pretreatment patient population with a histopathologic reference standard, be that biopsy or radical prostatectomy; they reported sufficient information to produce a 2 × 2 table (true positives, false positives, false negatives, and true negatives) for calculation of sensitivity and specificity; they were published in English; and they assessed more than ten individual patients. To be included, both T2WI and DWI sequences needed to be assessed visually, with both sequences used to assess for tumor presence rather than just for localization. The choice of scoring system, such as Likert or PI-RADS, and whether a sector-based or whole gland assessment was conducted did not affect eligibility. Articles were excluded if they did not satisfy the inclusion criteria above, or if they used a combination of imaging sequences other than DWI and T2WI so that individual data for the desired combination could not be extracted. They were also excluded if an ADC cutoff value was used to discriminate malignant from benign tissue as opposed to visual assessment by certified radiologists. Studies were not excluded by country of origin, age of patients or study design.
Study identification
Initially papers were reviewed by relevancy of title and then abstract. Residual articles had their full text reviewed against the inclusion and exclusion criteria. This was also done independently by the same two reviewers. Any disagreement was solved by consensus or a third expert reviewer if necessary.
Data extraction
The following data were extracted from each eligible study: year of publication, country of origin, patient group, number of patients, average age, and PSA, study design (retrospective or prospective) and the histopathologic reference standard used. Further information on the imaging specifications was also gathered: field strength, coil used, field-of-view, b-value set, and whether they visually assessed DWI source images, ADC maps, or both, for each patient. True positives, false positives, false negatives, and true negatives were also extracted for pooling results. In the case of multireader studies, the most experienced was chosen for data extraction. When insufficient data were available, reviewers manually calculated them from other reported statistics, when possible. All data extraction was independently verified by two reviewers.
Quality assessment
The quality of the individual included paper’s methodology was assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, a validated tool specifically designed to critically appraise diagnostic accuracy studies [15]. This was also undertaken independently by two reviewers and disagreement resolved with consensual discussion consulting a third expert reviewer if a consensus could not be met.
Statistical analysis
The sensitivity and specificity with 95% confidence intervals (CIs) were calculated for each included study using the extracted details of the 2 × 2 tables, and forest plots produced.
Initially, heterogeneity of studies was examined visually using the data extraction tables. Then, statistical analysis was performed using the inconsistency value (I 2) and Q statistics of the Chi squared value, for which an I 2 value > 50% or p value < 0.10, respectively, represents significant statistical heterogeneity. In these cases, a random-effects model was applied to data pooling. Pooled results for sensitivity, specificity, and diagnostic odds ratio (DOR) with 95% CIs, and a summary receiver-operating characteristic (sROC) curve were also presented.
To explore predictable sources of heterogeneity between the included studies, sensitivity and 1-specificity were plotted on an ROC plane to visually assess the presence or absence of a ‘shoulder arm’ shape, which indicates a threshold effect. This was also tested statistically with the Spearman correlation coefficient of the logit of sensitivity and logit of (1-specificity), with a p-value < 0.05 suggesting a threshold effect. Subgroup analysis was performed for; b-values (< 1000, 1000 and > 1000 s/mm2), field strength (1.5T and 3T), coil type (endorectal and body), method of assessment (DWI source images, ADC or both), reference standard (biopsy and radical prostatectomy), tumor zone (peripheral or transitional zone) and study design (retrospective and prospective). If possible raw data was separated from individual papers for each subgroup. Pooled sensitivities, specificities, positive and negative likelihood ratios, and meta-regression of diagnostic odds ratios were performed for these subgroups with a p-value < 0.05 deemed as statistically significant.
Publication bias was not assessed as there is currently no recognized or appropriate method that does so with sufficient power for diagnostic accuracy studies, and the impact of publication bias is presently unknown for studies of this type [16].
All statistical analysis was performed using Meta-DiSc (version 1.4, Javier Zamora).
Results
Search results
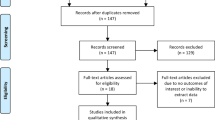
With the above-presented search strategy, 2825 citations were discovered, and after duplicates were removed, there were left 1880 unique articles. A total of 33 studies were included in the final analysis after reviewing against the eligibility criteria. The PRISMA flowchart of the search results is presented in Fig. 1.
Quality assessment
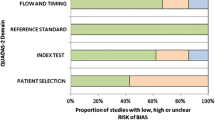
The full results of the QUADAS-2 appraisal are presented in Table 2. The strengths across the included studies were that the vast majority used consecutive patient selection with appropriate inclusion and exclusion criteria. However, two studies [17, 18] limited their investigation to transitional zone tumors and another [19] to patients with ‘low risk’ cancer. Therefore a subgroup analysis was deemed particularly important to assess the differences between peripheral and transitional zone tumors. Another strength was that all index tests used were applicable to clinical practice, without any nonstandard imaging methods. All but one study imaged patients after a positive biopsy, while patients studied by Tanimoto et al. had a pre-biopsy MRI [20]. A number of studies did not state the timings between biopsy and MRI [21,22,23,24], which could have implications if the timing was too long causing a disparity between the images and histopathology correlation or too short resulting in an increased incidence of post-biopsy hemorrhage which might limit accuracy. Kitajima et al. [25] and Morgan et al. [26] reported delays between biopsy and imaging much less than the recommended six weeks [27]. The predominant weakness of included studies was applicability of the patient groups, as studies were often limited to patients who underwent radical prostatectomy. These patients tend to be younger, with a narrower range of tumor staging. However, this is acceptable to obtain a reference test with low bias.
Study characteristics
The data extracted for study characteristics are described in Tables 3, 4, and 5. There were 2949 patients across the 33 studies. The mean age (range) was 65.1 (41–86) years, and PSA was 9 (0.4–130) ng/mL, respectively. The majority of studies (n = 20) used a retrospective study design as opposed to prospective (n = 13). Most of the studies (n = 19) used 3T field strength, thirteen studies used 1.5 T, and one study used both. Maximum b-values across the studies ranged from 600 to 2000 with the majority using 1000. Nine studies used an endorectal coil. Nine studies used DWI source images for diagnosis, while seven used ADC maps and seventeen used both. Most studies (n = 20) used radical prostatectomy as the reference standard while seven used TRUS biopsy, two MRI guided biopsy, one transperineal biopsy and another used a mixture of TRUS biopsy and radical prostatectomies.
Meta-analysis
Visual assessment of the data extraction tables indicated they were homogeneous enough to undertake a meta-analysis with pooling. The pooled sensitivity (Fig. 2) and specificity (Fig. 3) of all included studies were 0.69 (95% CI 0.68–0.69) and 0.84 (95% CI 0.83–0.85), respectively. The pooled DOR was 12.27 (95% CI 9.60–15.68). The sROC (Fig. 4) gave an AUC of 0.839, indicating good diagnostic accuracy.
The I 2 value and Chi-square Q were 94.6% and 882.53 (p < 0.001), respectively, for sensitivity and 96.7% and 1446.59 (p < 0.001) for specificity, indicating significant statistical heterogeneity. The ROC plane (Supplementary Fig. 1) did not show a ‘shoulder-arm’ shape; however, the Spearman rank coefficient of the logit of sensitivity against logit of (1-specificity) was 0.335 (p = 0.018), indicating there could be heterogeneity due to a threshold effect.
Sub-group analysis
The highest DORs were obtained when using ADC maps with or without DWI for tumors assessment and for b-values > 1000 s/mm2. Significantly higher sensitivity was achieved using b-values > 1000 s/mm2, 3T field strength, assessing PZ tumors, studies with a retrospective design and those using biopsy as a reference standard. Specificity improved significantly with a 1.5T field strength, assessing TZ tumors, using ADC maps with or without DWI and those studies using radical prostatectomy as the reference standard. The complete subgroup analysis is shown in Table 6.
Discussion
The findings from this study show the diagnostic accuracy of DWI and T2WI of prostate cancer is good when using visual assessment. The greatest diagnostic accuracy is achieved with b-values > 1000 s/mm2, and when assessing lesions with both DWI source images and ADC maps, although the interplay between sensitivity and specificity can be significantly altered by the choice of field strength and by whether tumors originate from the peripheral or transitional zone. The overall strength of the evidence on which this analysis was based was graded as good by the QUADAS-2 critical appraisal tool [15]. However, there was a high degree of unknown statistical heterogeneity, so care should be taken when interpreting these results, and even though this review cannot specify an optimal imaging protocol, it does highlight the likely important factors to be considered.
Our pooled results match those of meta-analyses investigating T2WI and DWI by Wu et al. and Tan et al.; this is likely due to the large overlap of included studies [28, 29]. Compared with Godley et al. and Jie et al. who analyzed the use of DWI alone, we observed a higher sensitivity but lower specificity [5, 30]. However, when we compare the results for just peripheral zone tumors, our pooled results are similar. This would suggest that the addition of T2WI improves the sensitivity for diagnosing transitional zone tumors; however, neither Godley nor Jie et al. presented a subgroup for TZ tumors or comparison [10]. This finding supports the present consensus that T2WI with DWI should be the predominant imaging protocol for diagnosing TZ tumors [9].
We observed a significant increase in sensitivity using a maximum b-value > 1000 s/mm2, and improved specificity with a maximum b-value of ≥ 1000 s/mm2. The improved contrast-to-noise ratio at higher b-values, resulting from the relative suppression of normal prostate tissue, would explain the increase in sensitivity by making tumors more visually apparent. Two of the studies [23, 24] also used computed high b-values. These synthetic data extrapolated from low b-value datasets showed relatively decreased sensitivity and increased specificity compared to the equivalent acquired b-values. There has been limited research comparing the diagnostic accuracy of computed DWI to standard DWI, but the method shows promise with reduced distortion and ghosting and improved tumor conspicuity [31, 32].
We also note that all studies using b-values > 1000 s/mm2 were limited to a maximum b-value of 2000 s/mm2, except the study by Kuhlet al [33]. Wang et al. and Metens et al. found b-values of 1500 s/mm2 gave a better tumor contrast and image quality than b-values of 1,000 or 2000 s/mm2and Kuhl et al. using a b-value of 1400 s/mm2, produced some of the highest sensitivities and specificities [33,34,35]. However, more data on the diagnostic accuracy of b ≈ 1,500 DWI are required. Furthermore, the maximum b-value, the minimum b-value, and the number of b-values have all been shown to have a strong influence on the calculated ADC values [36]. However, there is little evidence about their impact on diagnostic accuracy with visual assessment [10, 36].
All but two of the included studies in this analysis used b = 0 s/mm2 as the minimum b-value, but the number of b-values ranged from two to seven. Thörmer et al. found that using just two b-values and a minimum b-value of 50 s/mm2 gave an improved qualitative image score versus data with a minimum b-value of 0 s/mm2 [37]. However, they tested only a limited number of combinations, and used a maximum b-value of just 800 s/mm2. The significant heterogeneity of b-value choice in the included studies makes it extremely difficult to provide a conclusion that high b-values are indeed superior for diagnostic accuracy. The individual studies that tested multiple b-value sets on the same cohort do, however, show improved diagnostic accuracy using b = 2,000 as opposed to 1000 or lower. Further studies directly comparing b-value sets of different maximum, minimum, and a number of intermediary b-values would be required to make a stronger recommendation of b-value choice.
DOR was not significantly different between 1.5T and 3T studies (p = 0.418), but 3T studies showed a significantly higher sensitivity and significantly lower specificity than those performed at 1.5T. Higher field strengths have the advantage of increased SNR, which can be traded for better spatial and temporal resolutions; they also lead to increased susceptibility artifact and signal heterogeneity, and there is conflicting evidence with respect to the categorical advantage of 3T over 1.5 T [38]. There is a trend toward better diagnostic accuracy with 3T in our study, although this may be because these systems allow the use of higher b-values, which improve diagnostic accuracy. This result reflects the recommendations of PIRADS v2 that 1.5T and 3T are both adequate, but 3T is regarded optimal if available [9].
For a few of the studies, it was possible to separate the results for PZ and TZ, and we found significantly higher sensitivity for the PZ, but higher specificity for TZ. Often TZ tumors are of a lower grade than those found in the PZ, so they may be less apparent on imaging [39, 40]. There is also difficulty in differentiating malignancy from benign nodules common in the TZ, which are often heterogeneous and can demonstrate restricted diffusion. Along with the relative rarity of TZ tumors this may explain the drop in sensitivity but the overall DOR was not significantly different. It may be that different imaging parameters are needed for optimal diagnosis of peripheral or transitional zone disease.
Our results showed a significant increase in both sensitivity and specificity when using ADC maps with or without DWI source images for diagnostic assessment, as opposed to using DWI source images alone. There are many advantages to using ADC maps which might explain this change. Firstly, ADC maps give a quantitative measure of tissue diffusion, and are particularly useful in differentiating areas which have high signal on DWI images due to T2 shine-through, such as post-biopsy hemorrhage; this leads to reduced false positives and improved specificity versus weighted images. The ADC value can also be used to help confirm malignant lesions, which have low ADCs due to restricted diffusion, and this would explain the higher sensitivity seen.
Retrospective studies investigated men with previously confirmed prostate cancer, and therefore the readers knew there was cancer present in each prostate examined. This may cause the readers to be more liberal with diagnosing suspicious lesions in borderline cases where there were no other lesions in the gland, explaining the significantly higher sensitivity.
Using radical prostatectomy as the reference standard allows the assessment of individual tumors within the gland and is a more accurate method of defining tumor. TRUS biopsy is ‘blind’ and only samples a small area of the prostate, with a 20–30% false negative rate. This would lead to increased false positives on imaging, decreasing the specificity as we observe in the subgroup analysis.
This systematic review has a few limitations. Our search was, first, limited by a finite number of databases although those chosen contain the majority of the relevant journals, and by exploring the gray literature and hand-searching references, we believe the search strategy was of sufficient sensitivity. Specific databases for the research question were sought, but none existed. Second, the search was limited to the English language. The majority of articles are published in English, but there may be data in other languages that we did not include in this meta-analysis. We did not assess for publication bias for reasons stated in the statistical analysis section. The degree to which publication bias impacts diagnostic tests is unknown [16]. We did not review the exact T2WI parameters for the included studies, which could explain some of the heterogeneity seen. Reader experience is another factor which we did not assess as it was often poorly reported and in different formats such as years practicing, years reporting prostate mpMRI, or number of prostate mpMRIs. It is recognized that reader experience is important in interpreting mpMRI and should be considered when implementing prostate imaging [41, 42]. Although diagnostic accuracy is very important for prostate cancer assessment, there are other aims of mpMRI which have not been assessed in this meta-analysis: for example, assessment of extracapsular extension, seminal vesicle or lymph node involvement, and the ability of mpMRI to quantify tumor size and volume. These findings are all used in staging of disease and are relevant to decisions about optimal imaging sequences.
In conclusion, the diagnostic accuracy of combined diffusion- and T2-weighted magnetic resonance imaging for prostate cancer detection is good, and our results support the PI-RADS v2 guidelines [9]. The use of b-values > 1000 s/mm2 seem to improve the sensitivity while maintaining specificity. However, due to large amounts of heterogeneity, we cannot categorically recommend using maximum b-values up to 2000 s/mm2 for all DWI protocols for prostate cancer assessment. Further large-scale study investigating optimal b-value maximum, minimum, and number of b-values for the visual assessment of prostate cancer is required.
References
Office for National Statistics. Cancer Statistics: Registrations Series MB1. 2014
Cancer Research UK. Cancer Statistics April 2014
National Institute for Health and Clinical Excellence—Prostate cancer: diagnosis and management. 2014 Contract No.: CG175
Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN (2012) The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. Am J Roentgenol 199(1):103–110
Jie C, Rongbo L, Ping T (2014) The value of diffusion-weighted imaging in the detection of prostate cancer: a meta-analysis. Eur Radiol 24(8):1929–1941
Jin G, Su DK, Luo NB, et al. (2013) Meta-analysis of diffusion-weighted magnetic resonance imaging in detecting prostate cancer. J Comput Assist Tomogr 37(2):195–202
Tamada T, Sone T, Jo Y, et al. (2008) Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging 28(3):720–726
Barentsz JO, Richenberg J, Clements R, et al. (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757
Radiology ACo. Prostate Imaging—Reporting and Data System version 2. 2015.
Wang X, Qian Y, Liu B, et al. (2014) High-b-value diffusion-weighted MRI for the detection of prostate cancer at 3 T. Clin Radiol 69(11):1165–1170
Rosenkrantz AB, Hindman N, Lim RP, et al. (2013) Diffusion-weighted imaging of the prostate: comparison of b1000 and b2000 image sets for index lesion detection. J Magn Reson Imaging 38(3):694–700
Ueno Y, Kitajima K, Sugimura K, et al. (2013) Ultra-high b-value diffusion-weighted MRI for the detection of prostate cancer with 3-T MRI. J Magn Reson Imaging 38(1):154–160
National Institute for Health Reserach. PROSPERO, International prospective register of systematic reviews. http://www.crd.york.ac.uk/PROSPERO/.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Whiting PF, Rutjes AWS, Westwood ME, et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58(9):882–893
Hoeks CMA, Hambrock T, Yakar D, et al. (2013) Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 266(1):207–217
Jung IS, Donati OF, Vargas HA, et al. (2013) Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 269(2):493–503
Kim JY, Kim SH, Kim YH, et al. (2014) Low-risk prostate cancer: the accuracy of multiparametric MR imaging for detection. Radiology 271(2):435–444
Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S (2007) Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 25(1):146–152
Isebaert S, Van Den Bergh L, Haustermans K, et al. (2013) Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging 37(6):1392–1401
Petrillo A, Fusco R, Setola SV, et al. (2014) Multiparametric MRI for prostate cancer detection: performance in patients with prostate-specific antigen values between 2.5 and 10 ng/mL. J Magn Reson Imaging 39(5):1206–1212
Ueno Y, Takahashi S, Kitajima K, et al. (2013) Computed diffusion-weighted imaging using 3-T magnetic resonance imaging for prostate cancer diagnosis. Eur Radiol 23(12):3509–3516
Ueno Y, Takahashi S, Ohno Y, et al. (1048) Computed diffusion-weighted MRI for prostate cancer detection: The influence of the combinations of b-values. Br J Radiol 2015(88):20140738
Kitajima K, Kaji Y, Fukabori Y, et al. (2010) Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging 31(3):625–631
Morgan VA, Kyriazi S, Ashley SE, DeSouza NM (2007) Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol 48(6):695–703
Barrett T, Turkbey B, Choyke PL (2015) PI-RADS version 2: what you need to know. Clin Radiol 70(11):1165–1176
Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN (2012) The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. Am J Roentgenol 199(1):103–110
Tan CH, Wei W, Johnson V, Kundra V (2012) Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. Am J Roentgenol 199(4):822–829
Godley KC, Syer TJ, Toms AP, et al. (2017) Accuracy of high b-value diffusion-weighted MRI for prostate cancer detection: a meta-analysis. Acta Radiol. http://doi.org/10.1177/0284185117702181
Rosenkrantz AB, Chandarana H, Hindman N, et al. (2013) Computed diffusion-weighted imaging of the prostate at 3 T: impact on image quality and tumour detection. Eur Radiol 23(11):3170–3177
Vural M, Ertas G, Onay A, et al. (2014) Conspicuity of peripheral zone prostate cancer on computed diffusion-weighted imaging: comparison of cDWI1500, cDWI2000, and cDWI3000. Biomed Res Int 2014:768291
Kuhl C, Bruhn R, Krämer N, et al. (2017) Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology 000:170129
Metens T, Miranda D, Absil J, Matos C (2012) What is the optimal b value in diffusion-weighted MR imaging to depict prostate cancer at 3T? Eur Radiol 22(3):703–709
Wang X, Qian Y, Liu B, et al. (2014) High-b-value diffusion-weighted MRI for the detection of prostate cancer at 3 T. Clin Radiol 69(11):1165–1170
Peng Y, Jiang Y, Antic T, et al. (2014) Apparent diffusion coefficient for prostate cancer imaging: Impact of b values. Am J Roentgenol 202(3):W247–W253
Thormer G, Otto J, Horn LC, et al. (2015) Non-invasive estimation of prostate cancer aggressiveness using diffusion-weighted MRI and 3D proton MR spectroscopy at 3.0T. Acta Radiol 56(1):121–128
Kim CK, Park BK, Kim B (2010) Diffusion-weighted MRI at 3 T for the evaluation of prostate cancer. Am J Roentgenol 194(6):1461–1469
Augustin H, Erbersdobler A, Hammerer PG, Graefen M, Huland H (2004) Prostate cancers in the transition zone: part 2; clinical aspects. BJU Int 94(9):1226–1229
Greene DR, Wheeler TM, Egawa S, Weaver RP, Scardino PT (1991) Relationship between clinical stage and histological zone of origin in early prostate cancer: morphometric analysis. Br J Urol 68(5):499–509
Rosenkrantz AB, Lim RP, Haghighi M, et al. (2013) Comparison of interreader reproducibility of the prostate imaging reporting and data system and Likert scales for evaluation of multiparametric prostate MRI. Am J Roentgenol 201(4):W612–W618
Muller BG, Shih JH, Sankineni S, et al. (2015) Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric mr imaging1. Radiology 277(3):741–750
Agha M, Eid AF (2015) 3 Tesla MRI surface coil: is it sensitive for prostatic imaging? Alex J Med 51(2):111–119
Bains LJ, Studer UE, Froehlich JM, et al. (2014) Diffusion-weighted magnetic resonance imaging detects significant prostate cancer with high probability. J Urol 192(3):737–742
Baur A, Daqqaq T, Wagner M, et al. (2016) T2- and diffusion-weighted magnetic resonance imaging at 3T for the detection of prostate cancer with and without endorectal coil: an intraindividual comparison of image quality and diagnostic performance. Eur J Radiol 6(85):1075–1084
Brendle C, Martirosian P, Schwenzer N, et al. (2016) Diffusion-weighted imaging in the assessment of prostate cancer: comparison of zoomed imaging and conventional technique. Eur J Radiol 5(85):893–900
Costa D, Yuan Q, Xi Y, et al. (2016) Comparison of prostate cancer detection at 3-T MRI with and without an endorectal coil: a prospective, Paired-patient study. Urol Oncol 6(34):255
Doo KW, Sung DJ, Park BJ, et al. (2012) Detectability of low and intermediate or high risk prostate cancer with combined T2-weighted and diffusion-weighted MRI. Eur Radiol 22(8):1812–1819
Haider MA, Van Der Kwast TH, Tanguay J, et al. (2007) Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. Am J Roentgenol 189(2):323–328
Iwazawa J, Mitani T, Sassa S, Ohue S (2011) Prostate cancer detection with MRI: is dynamic contrast-enhanced imaging necessary in addition to diffusion-weighted imaging? Diagn Interv Radiol 17(3):243–248
Katahira K, Takahara T, Kwee TC, et al. (2011) Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 21(1):188–196
Lim HK, Kim JK, Kim KA, Cho K-S (2009) Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection–a multireader study. Radiology 250(1):145–151
Loggitsi D, Gyftopoulos A, Economopoulos N, et al. (2017) Multiparametric magnetic resonance imaging of the prostate for tumour detection and local staging: imaging in 1.5T and histopathologic correlation. Can Assoc Radiol J 68(4):379–386
Ohgiya Y, Suyama J, Seino N, et al. (2012) Diagnostic accuracy of ultra-high-b-value 3.0-T diffusion-weighted MR imaging for detection of prostate cancer. Clin Imaging 36(5):526–531
Rosenkrantz AB, Mannelli L, Kong X, et al. (2011) Prostate cancer: utility of fusion of T2-weighted and high b-value diffusion-weighted images for peripheral zone tumor detection and localization. J Magn Reson Imaging 34(1):95–100
Rosenkrantz AB, Kim S, Campbell N, et al. (2015) Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR Am J Roentgenol 204(3):W266–W272
Shimofusa R, Fujimoto H, Akamata H, et al. (2005) Diffusion-weighted imaging of prostate cancer. J Comput Assist Tomogr 29(2):149–153
Shinmoto H, Tamura C, Soga S, et al. (2015) Anterior prostate cancer: diagnostic performance of T2-weighted MRI and an apparent diffusion coefficient map. AJR Am J Roentgenol 205(2):W185–W192
Stanzione A, Imbriaco M, Cocozza S, et al. (2016) Biparametric 3T magnetic resonance imaging for prostatic cancer detection in a biopsy-naïve patient population: a further improvement of PI-RADS v2? Eur J Radiol 12(85):2269–2274
Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S (2007) Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 25(1):146–152
Thestrup K, Logager V, Baslev I, et al. (2016) Biparametric versus multiparametric MRI in the diagnosis of prostate cancer. Acta Radiol Open 8(5):205846011666304
Ueno Y, Takahashi S, Ohno Y, et al. (1048) Computed diffusion-weighted MRI for prostate cancer detection: the influence of the combinations of b-values. Br J Radiol 2015(88):20140738
Vargas HA, Akin O, Franiel T, Mazaheri Y, et al. (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259(3):775–784
Yoshimitsu K, Kiyoshima K, Irie H, et al. (2008) Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging 27(1):132–139
Yoshizako T, Wada A, Hayashi T, et al. (2008) Usefulness of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate transition-zone cancer. Acta Radiol 49(10):1207–1213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Author Tom Syer declares he has no conflict of interest. Author Keith Godley declares he has no conflict of interest. Author Donnie Cameron declares he has no conflict of interest. Author Paul Malcolm declares he has no conflict of interest.
Electronic supplementary material
Supplementary Figure 1. Receiver operating characteristics (ROC) plane, indicating the absence of a ‘shoulder arm’ shape. Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Syer, T.J., Godley, K.C., Cameron, D. et al. The diagnostic accuracy of high b-value diffusion- and T2-weighted imaging for the detection of prostate cancer: a meta-analysis. Abdom Radiol 43, 1787–1797 (2018). https://doi.org/10.1007/s00261-017-1400-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1400-4