Abstract
Purpose
To correlate RECIST, volumetric criteria, and tumor growth kinetics at multidetector-computed tomography with tumor metabolic activity at FDG PET in colorectal liver metastases (CRCLM) treated with bevacizumab-based chemotherapy.
Methods
Thirty-two CRCLM in 20 patients treated with bevacizumab-based chemotherapy were evaluated. Pre- and post-treatment CT scans were used to calculate reciprocal of doubling time (RDT), percentage change in the lesion’s longest transaxial diameter (RECIST 1.1), and percentage change in the tumor volume. The accuracy of these parameters in predicting response based on standard uptake value analysis at FDG PET was assessed. Data were analyzed using Spearman’s correlation, student’s t, Mann–Whitney, Wilcoxon signed-rank, and Fisher’s exact tests.
Results
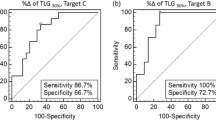
According to FDG PET, 24/32 (75%) lesions were categorized as responders and 8/32 (25%) lesions as nonresponders. Based on RDT, 26/32 (81.25%) lesions were classified as responders and 6/32 (18.75%) lesions as nonresponders. Response classification according to RDT and FDG PET was concordant in 30/32 (93.75%) lesions, whereas RECIST 1.1 and volumetric criteria were concordant with FDG PET for 20/32 (62.5%) and 21/32 (65.63%) lesions, respectively. A strong association was found between RDT and response based on FDG PET (odds ratio = 127.4; 95% CI 5.54–2997; P < 0.0001).
Conclusions
Tumor growth kinetics may be an effective imaging biomarker for response evaluation in CRCLM.



Similar content being viewed by others
Abbreviations
- WHO:
-
World Health Organization
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- 18F FDG:
-
18-Fluoro-2-deoxyglucose
- PET:
-
Positron emission tomography
- CRCLM:
-
Colorectal liver metastases
- MDCT:
-
Multidetector-computed tomography
- SUV:
-
Standard uptake value
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- RDT:
-
Reciprocal of doubling time
References
Lee HS, Kim HO, Hong YS, et al. (2014) Prognostic value of metabolic parameters in patients with synchronous colorectal cancer liver metastasis following curative-intent colorectal and hepatic surgery. J Nucl Med 55(4):582–589. doi:10.2967/jnumed.113.128629
Tochetto SM, Rezai P, Rezvani M, et al. (2010) Does multidetector CT attenuation change in colon cancer liver metastases treated with 90Y help predict metabolic activity at FDG PET? Radiology 255(1):164–172. doi:10.1148/radiol.09091028
Chung WS, Park MS, Shin SJ, et al. (2012) Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol 199(4):809–815. doi:10.2214/AJR.11.7910
Zoratto F, Rossi L, Zullo A, et al. (2012) Critical appraisal of bevacizumab in the treatment of metastatic colorectal cancer. Onco Targets Ther 5:199–211. doi:10.2147/OTT.S30581
Organization WH (1979) WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization
Therasse P, Arbuck SG, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Seyal AR, Parekh K, Velichko YS, Salem R, Yaghmai V (2014) Tumor growth kinetics versus RECIST to assess response to locoregional therapy in breast cancer liver metastases. Acad Radiol. doi:10.1016/j.acra.2014.02.015
Tirkes T, Hollar MA, Tann M, et al. (2013) Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 33(5):1323–1341. doi:10.1148/rg.335125214
Goshen E, Davidson T, Zwas ST, Aderka D (2006) PET/CT in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol Cancer Res Treat 5(1):37–43
Lastoria S, Piccirillo MC, Caraco C, et al. (2013) Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med 54(12):2062–2069. doi:10.2967/jnumed.113.119909
Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Kuhnigk JM, Dicken V, Bornemann L, et al. (2006) Morphological segmentation and partial volume analysis for volumetry of solid pulmonary lesions in thoracic CT scans. IEEE Trans Med Imaging 25(4):417–434. doi:10.1109/TMI.2006.871547
Moltz JH, Bornemann L, Kuhnigk J-M, et al. (2009) Advanced segmentation techniques for lung nodules, liver metastases, and enlarged lymph nodes in CT scans. IEEE J Sel Top Signal Process 3(1):122–134. doi:10.1109/JSTSP.2008.2011107
Keil S, Behrendt FF, Stanzel S, et al. (2008) Semi-automated measurement of hyperdense, hypodense and heterogeneous hepatic metastasis on standard MDCT slices. Comparison of semi-automated and manual measurement of RECIST and WHO criteria. Eur Radiol 18(11):2456–2465. doi:10.1007/s00330-008-1050-6
Soyer P, Poccard M, Boudiaf M, et al. (2004) Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology 231(2):413–420. doi:10.1148/radiol.2312021639
Chalian H, Tochetto SM, Tore HG, Rezai P, Yaghmai V (2012) Hepatic tumors: region-of-interest versus volumetric analysis for quantification of attenuation at CT. Radiology 262(3):853–861. doi:10.1148/radiol.11110106
Shankar LK, Hoffman JM, Bacharach S, et al. (2006) Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 47(6):1059–1066
Young H, Baum R, Cremerius U, et al. (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35(13):1773–1782
Eaton BR, Kim HS, Schreibmann E, et al. (2014) Quantitative dosimetry for yttrium-90 radionuclide therapy: tumor dose predicts fluorodeoxyglucose positron emission tomography response in hepatic metastatic melanoma. J Vasc Interv Radiol 25(2):288–295. doi:10.1016/j.jvir.2013.08.021
Skougaard K, Nielsen D, Jensen BV, Hendel HW (2013) Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med 54(7):1026–1031. doi:10.2967/jnumed.112.111757
Zerizer I, Al-Nahhas A, Towey D, et al. (2012) The role of early (1)(8)F-FDG PET/CT in prediction of progression-free survival after (9)(0)Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 39(9):1391–1399. doi:10.1007/s00259-012-2149-1
Prasad SR, Jhaveri KS, Saini S, et al. (2002) CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology 225(2):416–419. doi:10.1148/radiol.2252011604
Schwartz M (1961) A biomathematical approach to clinical tumor growth. Cancer 14:1272–1294
Keil S, Plumhans C, Behrendt FF, et al. (2009) Semi-automated quantification of hepatic lesions in a phantom. Invest Radiol 44(2):82–88. doi:10.1097/RLI.0b013e3181911ffa
Altman DG (1991) Practical statistics for medical research, 1st edn. London: Chapman & Hall
Gonzalez-Guindalini FD, Botelho MP, Harmath CB, et al. (2013) Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics 33(6):1781–1800. doi:10.1148/rg.336135511
Shankar LK, Van den Abbeele A, Yap J, et al. (2009) Considerations for the use of imaging tools for phase II treatment trials in oncology. Clin Cancer Res 15(6):1891–1897. doi:10.1158/1078-0432.CCR-08-2030
Dhani N, Tu D, Sargent DJ, Seymour L, Moore MJ (2009) Alternate endpoints for screening phase II studies. Clin Cancer Res 15(6):1873–1882. doi:10.1158/1078-0432.CCR-08-2034
Shindoh J, Chun YS, Loyer EM, Vauthey JN (2013) Non-size-based response criteria to preoperative chemotherapy in patients with colorectal liver metastases: the morphologic response criteria. Curr Colorectal Cancer Rep 9(2):198–202. doi:10.1007/s11888-013-0164-7
Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ (2002) Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging 29(6):815–820. doi:10.1007/s00259-002-0787-4
El Sharouni SY, Kal HB, Battermann JJ (2003) Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br J Cancer 89(12):2184–2189. doi:10.1038/sj.bjc.6601418
Stein WD, Yang J, Bates SE, Fojo T (2008) Bevacizumab reduces the growth rate constants of renal carcinomas: a novel algorithm suggests early discontinuation of bevacizumab resulted in a lack of survival advantage. Oncologist 13(10):1055–1062. doi:10.1634/theoncologist.2008-0016
Rezai P, Yaghmai V, Tochetto SM, et al. (2011) Change in the growth rate of localized pancreatic adenocarcinoma in response to gemcitabine, bevacizumab, and radiation therapy on MDCT. Int J Radiat Oncol Biol Phys 81(2):452–459. doi:10.1016/j.ijrobp.2010.05.060
Wulff AM, Bolte H, Fischer S, et al. (2012) Lung, liver and lymph node metastases in follow-up MSCT: comprehensive volumetric assessment of lesion size changes. Rofo 184(9):820–828. doi:10.1055/s-0032-1312860
Acknowledgment
Adeel R. Seyal, Keyur Parekh, and Atilla Arslanoglu received educational Grant from Siemens Healthcare.
Conflict of interest
All other authors had nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seyal, A.R., Parekh, K., Arslanoglu, A. et al. Performance of tumor growth kinetics as an imaging biomarker for response assessment in colorectal liver metastases: correlation with FDG PET. Abdom Imaging 40, 3043–3051 (2015). https://doi.org/10.1007/s00261-015-0546-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0546-1