Abstract
Purpose
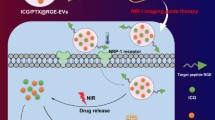
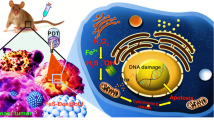
Photodynamic therapy (PDT) is a promising cancer treatment strategy with rapid progress in preclinical and clinical settings. However, the limitations in penetration of external light and precise delivery of photosensitizers hamper its clinical translation. As such, the internal light source such as Cerenkov luminescence (CL) from decaying radioisotopes offers new opportunities. Herein, we show that goat milk-derived extracellular vesicles (GEV) can act as a carrier to deliver photosensitizer Chlorin e6 (Ce6) and tumor-avid 18F-FDG can activate CL-induced PDT for precision cancer theranostics.
Methods
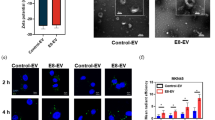
GEV was isolated via differential ultracentrifugation of commercial goat milk and photosensitizer Ce6 was loaded by co-incubation to obtain Ce6@GEV. Tumor uptake of Ce6@GEV was examined using confocal microscopy and flow cytometry. To demonstrate the ability of 18F-FDG to activate photodynamic effects against cancer cells, apoptosis rates were measured using flow cytometry, and the production of 1O2 was measured by reactive oxygen species (ROS) monitoring kit. Moreover, we used the IVIS device to detect Cherenkov radiation and Cerenkov radiation energy transfer (CRET). For animal experiments, a small-animal IVIS imaging system was used to visualize the accumulation of the GEV drug delivery system in tumors. PET/CT and CL images of the tumor site were performed at 0.5, 1, and 2 h. For in vivo antitumor therapy, changes of tumor volume, survival time, and body weight in six groups of tumor-bearing mice were monitored. Furthermore, the blood sample and organs of interest (heart, liver, spleen, lungs, kidneys, and tumor) were collected for hematological analysis, immunohistochemistry, and H&E staining.
Results
Confocal microscopy of 4T1 cells incubated with Ce6@GEV for 4 h revealed strong red fluorescence signals in the cytoplasm, which demonstrated that Ce6 loaded in GEV could be efficiently delivered into tumor cells. When Ce6@GEV and 18F-FDG co-existed incubated with 4T1 cells, the cell viability plummeted from more than 88.02 ± 1.30% to 23.79 ± 1.59%, indicating excellent CL-induced PDT effects. In vivo fluorescence images showed a peak tumor/liver ratio of 1.36 ± 0.09 at 24 h after Ce6@GEV injection. For in vivo antitumor therapy, Ce6@GEV + 18F-FDG group had the best tumor inhibition rate (58.02%) compared with the other groups, with the longest survival rate (35 days, 40%). During the whole treatment process, neither blood biochemical analysis nor histological observation revealed vital organ damage, suggesting the biosafety of this treatment strategy.
Conclusions
The simultaneous accumulation of 18F-FDG and Ce6 in tumor tissues is expected to overcome the deficiency of traditional PDT. This strategy has the potential to extend PDT to a variety of tumors, including metastases, using targeted radiotracers to provide internal excitation of light-responsive therapeutics. We expect that our method will play a critical role in precision treatment of deep solid tumors.








Similar content being viewed by others
Abbreviations
- PDT :
-
photodynamic therapy
- CL :
-
Cerenkov luminescence
- UV :
-
ultraviolet
- EVs :
-
extracellular vesicles
- GEV :
-
goat milk-derived extracellular vesicles
- PS :
-
photosensitizers
- ROS :
-
reactive oxygen species
- 1 O 2 :
-
singlet oxygen
- CRET :
-
Cerenkov radiation energy transfer
- 18 F-FDG :
-
18F-fluo-rodeoxyglucose
- Ce6 :
-
Chlorin e6
- FBS :
-
fetal bovine serum
- PBS :
-
phosphate buffer saline
- TEM :
-
transmission electron microscope
- DLS :
-
dynamic light scattering
- WB :
-
Western blot
- IVIS :
-
In Vivo Imaging System
- i.v. :
-
intravenous injection
- p.i. :
-
post-injection
- ALT :
-
alanine aminotransferase
- AST :
-
aspartate aminotransferase
- ALP :
-
alkaline phosphatase
- BUN :
-
blood urea nitrogen
- CRE :
-
creatinine
- DDS :
-
drug delivery systems
- EPR effect :
-
enhanced permeability and retention effect
References
Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7.
Liu S, Feng G, Tang BZ, Liu B. Recent advances of AIE light-up probes for photodynamic therapy. Chem Sci. 2021;12:6488–506. https://doi.org/10.1039/d1sc00045d.
Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec. 2017;17:775–802. https://doi.org/10.1002/tcr.201600121.
Grigalavicius M, Mastrangelopoulou M, Berg K, Arous D, Ménard M, Raabe-Henriksen T, et al. Proton-dynamic therapy following photosensitiser activation by accelerated protons demonstrated through fluorescence and singlet oxygen production. Nat Commun. 2019;10:3986. https://doi.org/10.1038/s41467-019-12042-7.
Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–74. https://doi.org/10.1038/s41571-020-0410-2.
Blum NT, Zhang Y, Qu J, Lin J, Huang P. Recent advances in self-exciting photodynamic therapy. Front Bioeng Biotechnol. 2020;8:594491. https://doi.org/10.3389/fbioe.2020.594491.
Kavadiya S, Biswas P. Design of Cerenkov radiation-assisted photoactivation of TiO nanoparticles and reactive oxygen species generation for cancer treatment. J Nucl Med. 2019;60:702–9. https://doi.org/10.2967/jnumed.118.215608.
Shaffer TM, Pratt EC, Grimm J. Utilizing the power of Cerenkov light with nanotechnology. Nat Nanotechnol. 2017;12:106–17. https://doi.org/10.1038/nnano.2016.301.
Shaffer TM, Drain CM, Grimm J. Optical imaging of ionizing radiation from clinical sources. J Nucl Med. 2016;57:1661–6.
Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–9. https://doi.org/10.1038/nnano.2015.17.
Kamkaew A, Chen F, Zhan Y, Majewski RL, Cai W. Scintillating nanoparticles as energy mediators for enhanced photodynamic therapy. ACS Nano. 2016;10:3918–35. https://doi.org/10.1021/acsnano.6b01401.
Ni D, Ferreira CA, Barnhart TE, Quach V, Yu B, Jiang D, et al. Magnetic targeting of nanotheranostics enhances Cerenkov radiation-induced photodynamic therapy. J Am Chem Soc. 2018;140:14971–9. https://doi.org/10.1021/jacs.8b09374.
Lioret V, Bellaye P-S, Arnould C, Collin B, Decréau RA. Dual Cherenkov radiation-induced near-infrared luminescence imaging and photodynamic therapy toward tumor resection. J Med Chem. 2020;63:9446–56. https://doi.org/10.1021/acs.jmedchem.0c00625.
Zhang Y, Hao Y, Chen S, Xu M. Photodynamic therapy of cancers with internal light sources: chemiluminescence, bioluminescence, and Cerenkov radiation. Front Chem. 2020;8:770. https://doi.org/10.3389/fchem.2020.00770.
Wang R, Li X, Yoon J. Organelle-targeted photosensitizers for precision photodynamic therapy. ACS Appl Mater Interfaces. 2021;13:19543–71. https://doi.org/10.1021/acsami.1c02019.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96. https://doi.org/10.1016/j.apsb.2016.02.001.
van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022. https://doi.org/10.1038/s41580-022-00460-3.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Wiklander OPB, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. https://doi.org/10.3402/jev.v4.26316.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga A-H, Wilcher SA, et al. Milk exosomes - natural nanoparticles for siRNA delivery. Cancer Lett. 2019;449:186–95. https://doi.org/10.1016/j.canlet.2019.02.011.
Mecocci S, Pietrucci D, Milanesi M, Pascucci L, Filippi S, Rosato V, et al. Transcriptomic Characterization of cow, donkey and goat milk extracellular vesicles reveals their anti-inflammatory and immunomodulatory potential. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms222312759.
Yuan A, Tang X, Qiu X, Jiang K, Wu J, Hu Y. Activatable photodynamic destruction of cancer cells by NIR dye/photosensitizer loaded liposomes. Chem Commun (Camb). 2015;51:3340-2. https://doi.org/10.1039/c4cc09689d.
Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, et al. Hollow MnO as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902. https://doi.org/10.1038/s41467-017-01050-0.
Cheng L, Kamkaew A, Sun H, Jiang D, Valdovinos HF, Gong H, et al. Dual-modality positron emission tomography/optical image-guided photodynamic cancer therapy with chlorin e6-containing nanomicelles. ACS Nano. 2016;10:7721–30. https://doi.org/10.1021/acsnano.6b03074.
Lee W, Jeon M, Choi J, Oh C, Kim G, Jung S, et al. Europium-diethylenetriaminepentaacetic acid loaded radioluminescence liposome nanoplatform for effective radioisotope-mediated photodynamic therapy. ACS Nano. 2020;14:13004–15. https://doi.org/10.1021/acsnano.0c04324.
Kamkaew A, Cheng L, Goel S, Valdovinos HF, Barnhart TE, Liu Z, et al. Cerenkov radiation induced photodynamic therapy using chlorin e6-loaded hollow mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2016;8:26630–7.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14. https://doi.org/10.1038/mt.2010.105.
Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS ONE. 2010;5:e13300. https://doi.org/10.1371/journal.pone.0013300.
Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355–N65. https://doi.org/10.1088/0031-9155/54/16/N01.
Xu Y, Liu H, Cheng Z. Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J Nucl Med. 2011;52:2009–18. https://doi.org/10.2967/jnumed.111.092965.
Cheng L, Jiang D, Kamkaew A, Valdovinos HF, Im H-J, Feng L, et al. Renal-clearable PEGylated porphyrin nanoparticles for image-guided photodynamic cancer therapy. Adv Funct Mater. 2017;27. https://doi.org/10.1002/adfm.201702928.
Xia Q, Zhang Y, Li Z, Hou X, Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9:675–89. https://doi.org/10.1016/j.apsb.2019.01.011.
Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. https://doi.org/10.1016/j.addr.2021.113851.
Moghassemi S, Dadashzadeh A, Azevedo RB, Feron O, Amorim CA. Photodynamic cancer therapy using liposomes as an advanced vesicular photosensitizer delivery system. J Control Release. 2021;339:75–90. https://doi.org/10.1016/j.jconrel.2021.09.024.
Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45. https://doi.org/10.1016/j.drup.2019.07.003.
He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–55. https://doi.org/10.7150/thno.21945.
Santos-Coquillat A, González MI, Clemente-Moragón A, González-Arjona M, Albaladejo-García V, Peinado H, et al. Goat milk exosomes as natural nanoparticles for detecting inflammatory processes by optical imaging. Small. 2022;18:e2105421. https://doi.org/10.1002/smll.202105421.
Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. https://doi.org/10.1016/j.canlet.2015.10.020.
Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. https://doi.org/10.3402/jev.v3.24641.
Qian R, Jing B, Jiang D, Gai Y, Zhu Z, Huang X, et al. Multi-antitumor therapy and synchronous imaging monitoring based on exosome. Eur J Nucl Med Mol Imaging. 2022. https://doi.org/10.1007/s00259-022-05696-x.
Jing B, Gai Y, Qian R, Liu Z, Zhu Z, Gao Y, et al. Hydrophobic insertion-based engineering of tumor cell-derived exosomes for SPECT/NIRF imaging of colon cancer. J Nanobiotechnology. 2021;19:7. https://doi.org/10.1186/s12951-020-00746-8.
Gill RK, Mitchell GS, Cherry SR. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys Med Biol. 2015;60:4263–80. https://doi.org/10.1088/0031-9155/60/11/4263.
Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–48. https://doi.org/10.1016/j.jconrel.2016.01.020.
Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021;12:1864. https://doi.org/10.1038/s41467-021-22126-y.
Acknowledgements
We thank colleagues of the Key Laboratory of Molecular Imaging for discussions and comments on this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82102121, 82071966, and 81873904), and thanks to the Open Foundation of Hubei Provincial Key Laboratory of Molecular Imaging (No. 2021fzyx009).
Author information
Authors and Affiliations
Contributions
Conceptualization, R. Guo, D. Jiang, and Y. Gai; methodology, R. Guo, R. Qian, Z. Zhu, and Y. Gao; formal analysis, B. Jing and B. Yang; investigation, X. Lan and R. An; writing–original draft, R. Guo and R. An; writing–review and editing, R. An, D. Jiang, and X. Lan; project administration, R. An, D. Jiang, and X. Lan; funding acquisition, Y. Gai, D. Jiang, R. An, and X. Lan.
Corresponding authors
Ethics declarations
Ethics approval
All animal experiment protocols were reviewed and approved by the Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology. BALB/c mice (female, 4–5 weeks old, Beijing Vital River Laboratory Animal Technology Co., Ltd, China) fed in a pathogen-free environment.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic
Supplementary information
ESM 1
(DOCX 3084 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, R., Jiang, D., Gai, Y. et al. Chlorin e6-loaded goat milk-derived extracellular vesicles for Cerenkov luminescence-induced photodynamic therapy. Eur J Nucl Med Mol Imaging 50, 508–524 (2023). https://doi.org/10.1007/s00259-022-05978-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05978-4