Abstract
Purpose
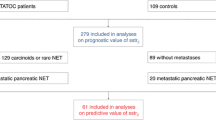
We aimed to elucidate the role of quantitative tumor burden based on PET/CT of somatostatin receptors in well-differentiated neuroendocrine tumors (NETs).
Methods
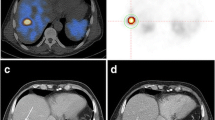
This study enrolled patients with [68 Ga]Ga-DOTA-NOC PET/CT-positive advanced NETs who did not receive medical treatment prior to PET/CT. Tumor burden was calculated using methods based on the background threshold and relative fixed threshold values (30%, 40%, and 50%). The prognostic value of the measured tumor burden in reference to overall survival (OS) and progression-free survival (PFS) on treatment with octreotide long-acting repeatable (LAR) was assessed using Cox regression analysis, Harrell’s C-index, and survival analysis. A classification and regression tree (CART) was used to determine the optimal threshold for tumor burden.
Results
A total of 204 patients were included. Somatostatin receptor-expressing tumor volume (SRETV) and liver SRETV derived from a relative fixed threshold of 30% (SRETV30 and liver SRETV30) were statistically significantly associated with OS (C-index: 0.802 [95% confidence interval (CI), 0.658–0.946] and 0.806 [95% CI, 0.664–0.948], respectively). Extrahepatic tumor burden was not correlated with OS (hazard ratio: 0.617, 95% CI: 0.241–1.574, P = 0.312). Among 155 patients with non-functional NETs with a ki-67 index of ≤ 10%, those with a high SRETV30 (P = 0.016) or high liver SRETV30 (P = 0.014) showed statistically significantly worse PFS on treatment with octreotide LAR. Patients receiving a higher dose of octreotide LAR normalized by SRETV30 or liver SRETV30 (a normalized dose or a liver normalized dose) showed prolonged PFS on treatment with octreotide LAR and a prolonged OS.
Conclusion
Quantitative tumor burden based on [68 Ga]Ga-DOTA-NOC PET/CT was correlated with OS and PFS in patients with non-functional NETs with a ki-67 index of ≤ 10% who received octreotide LAR. Calculating normalized and liver normalized doses may help in selecting the starting dose of octreotide LAR.




Similar content being viewed by others
Data availability
The clinical data and imaging files used in this study, though not available in a public repository, are available from the corresponding authors upon reasonable request.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. https://doi.org/10.1001/jamaoncol.2017.0589.
Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–85. https://doi.org/10.1159/000443167.
Pavel M, Oberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844–60. https://doi.org/10.1016/j.annonc.2020.03.304.
Baudin E, Caplin M, Garcia-Carbonero R, Fazio N, Ferolla P, Filosso PL, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(). Ann Oncol. 2021;32:439–51. https://doi.org/10.1016/j.annonc.2021.01.003.
Ortega C, Wong RK, Schaefferkoetter J, Veit-Haibach P, Myrehaug S, Juergens R, et al. Quantitative (68)Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with (177)Lu-DOTATATE. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.120.256727.
Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33. https://doi.org/10.1056/NEJMoa1316158.
Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76. https://doi.org/10.1159/000335597.
Shen C, Xu Y, Dasari A, Shih YC, Yao JC. Octreotide LAR dosage and survival among elderly patients with distant-stage neuroendocrine tumors. Oncologist. 2016;21:308–13. https://doi.org/10.1634/theoncologist.2015-0381.
Broder MS. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: a systematic literature review. World J Gastroenterol. 2015;21:1945. https://doi.org/10.3748/wjg.v21.i6.1945.
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. https://doi.org/10.1200/jco.2009.22.8510.
Toriihara A, Baratto L, Nobashi T, Park S, Hatami N, Davidzon G, et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:2244–51. https://doi.org/10.1007/s00259-019-04455-9.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-S150. https://doi.org/10.2967/jnumed.108.057307.
Carlsen EA, Johnbeck CB, Loft M, Pfeifer A, Oturai P, Langer SW, et al. Semi-automatic tumor delineation for evaluation of (64)Cu-DOTATATE PET/CT in patients with neuroendocrine neoplasms: prognostication based on lowest lesion uptake and total tumor volume. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.120.258392.
Pettinato C, Sarnelli A, Di Donna M, Civollani S, Nanni C, Montini G, et al. 68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2008;35:72–9. https://doi.org/10.1007/s00259-007-0587-y.
Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging. 2018;52:5–15. https://doi.org/10.1007/s13139-017-0493-6.
Tirosh A, Papadakis GZ, Millo C, Hammoud D, Sadowski SM, Herscovitch P, et al. Prognostic utility of total (68)Ga-DOTATATE-Avid tumor volume in patients with neuroendocrine tumors. Gastroenterology. 2018;154:998-1008.e1. https://doi.org/10.1053/j.gastro.2017.11.008.
Heidari P, Wehrenberg-Klee E, Habibollahi P, Yokell D, Kulke M, Mahmood U. Free somatostatin receptor fraction predicts the antiproliferative effect of octreotide in a neuroendocrine tumor model: implications for dose optimization. Can Res. 2013;73:6865–73. https://doi.org/10.1158/0008-5472.can-13-1199.
Wild D, Bomanji JB, Benkert P, Maecke H, Ell PJ, Reubi JC, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54:364–72. https://doi.org/10.2967/jnumed.112.111724.
Acknowledgements
This study was supported by the Guangdong Provincial Basic and Applied Basic Research Fund (grant no. 2021A1515110241). This funder had no role in the design, conduct, or reporting of this work.
Funding
This study was supported by the Guangdong Provincial Basic and Applied Basic Research Fund (grant no. 2021A1515110241). This funder had no role in the design, conduct, or reporting of this work.
Author information
Authors and Affiliations
Contributions
Luohai Chen, Nuerailaguli Jumai, Xiangsong Zhang, and Ning Zhang each contributed to the study conception and design. Data collection was performed by Luohai Chen, Nuerailaguli Jumai, and Qiao He. Data analysis and interpretation of results was also conducted by Luohai Chen, Nuerailaguli Jumai, and Qiao He. Luohai Chen drafted the manuscript, and Nuerailaguli Jumai, Qiao He, Man Liu, Yuan Lin, Yu Wang, Min-hu Chen, and Zhirong Zeng each contributed to the critical revision of the manuscript. All authors reviewed the manuscript and provided approval for the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the ethics committee of The First Affiliated Hospital, Sun Yat-sen University (approval no. 2022–062) and was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
The requirement for formal consent was waived by the approving ethics committee due to the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—General
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Jumai, N., He, Q. et al. The role of quantitative tumor burden based on [68 Ga]Ga-DOTA-NOC PET/CT in well-differentiated neuroendocrine tumors: beyond prognosis. Eur J Nucl Med Mol Imaging 50, 525–534 (2023). https://doi.org/10.1007/s00259-022-05971-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05971-x