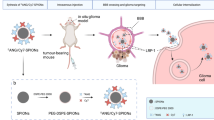
Abstract
Purpose
The surgery of glioblastoma (GBM) requires a maximal resection of the tumor when it is safe and feasible. The infiltrating growth property of the GBM makes it a challenge for neurosurgeons to identify the tumor tissue even with the assistance of the surgical microscope. This highlights the urgent requirement for imaging techniques that can differentiate tumor tissues during surgery in real time. Fluorescence image-guided surgery of GBM has been investigated using several non-specific fluorescent probes that emit light in the visible and the first near-infrared window (NIR-I, 700–900 nm), which limit the detection accuracy because of the non-specific targeting mechanism and spectral characteristics. Targeted NIR-II (1000–1700 nm) fluorescent probes for GBM are thus highly desired. The folate receptor (FR) has been reported to be upregulated in GBM, which renders it to be a promising target for specific tumor imaging.
Methods
In this study, the folic acid (FA) that can target the FR was conjugated with the clinically approved indocyanine green (ICG) dye and DOTA chelator for radiolabeling with 64Cu to achieve targeted positron emission tomography (PET) and fluorescence imaging of GBM.
Results
Surprisingly it was found that the resulted bioconjugate, DOTA-FA-ICG and non-radioactive natCu-DOTA-FA-ICG, were both self-assembled into nanoparticles with NIR-II emission signal. The radiolabeled DOTA-FA-ICG, 64Cu-DOTA-FA-ICG, was found to specifically accumulate in the orthotopic GBM models using in vivo PET, NIR-II, and NIR-I fluorescence imaging. The best time window of fluorescence imaging was demonstrated to be 24 h after DOTA-FA-ICG injection. NIR-II fluorescence image-guided surgery was successfully conducted in the orthotopic GBM models using DOTA-FA-ICG. All the fluorescent tissue was removed and proved to be GBM by the H&E examination.
Conclusion
Overall, our study demonstrates that the probes, 64Cu-DOTA-FA-ICG and DOTA-FA-ICG, hold promise for preoperative PET examination and intraoperative NIR-II fluorescence image-guided surgery of GBM, respectively.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data generated or analyzed during this study are included in the manuscript and the supplementary information files.
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-Oncol. 2020;22(Supplement_1):iv1–96. https://doi.org/10.1093/neuonc/noaa269.
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions. CA: A Cancer J Clin. 2020;70(4):299–312. https://doi.org/10.3322/caac.21613.
Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–9. https://doi.org/10.1001/jamaoncol.2016.1373.
Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, et al. Association of maximal extent of resection of contrast-enhanced and non–contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495–503. https://doi.org/10.1001/jamaoncol.2019.6143.
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710–9. https://doi.org/10.1212/WNL.0000000000001262.
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. https://doi.org/10.1016/S1470-2045(06)70665-9.
Wei L, Roberts DW, Sanai N, Liu JTC. Visualization technologies for 5-ALA-based fluorescence-guided surgeries. J Neurooncol. 2019;141(3):495–505. https://doi.org/10.1007/s11060-018-03077-9.
Bowden SG, Neira JA, Gill BJA, Ung TH, Englander ZK, Zanazzi G, et al. Sodium fluorescein facilitates guided sampling of diagnostic tumor tissue in nonenhancing gliomas. Neurosurgery. 2018;82(5):719–27. https://doi.org/10.1093/neuros/nyx271.
Acerbi F, Broggi M, Schebesch KM, Höhne J, Cavallo C, De Laurentis C, et al. Fluorescein-guided surgery for resection of high-grade gliomas: a multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res. 2018;24(1):52–61. https://doi.org/10.1158/1078-0432.CCR-17-1184.
Lee JYK, Thawani JP, Pierce J, Zeh R, Martinez-Lage M, Chanin M, et al. Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery. 2016;79(6):856–71. https://doi.org/10.1227/NEU.0000000000001450.
Cho SS, Sheikh S, Teng CW, Georges J, Yang AI, De Ravin E, et al. Evaluation of diagnostic accuracy following the coadministration of delta-aminolevulinic acid and second window indocyanine green in rodent and human glioblastomas. Mol Imag Biol. 2020;22:1266–79. https://doi.org/10.1007/s11307-020-01504-w.
Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20(7):e354–67. https://doi.org/10.1016/S1470-2045(19)30317-1.
Zhang RR, Schroeder AB, Grudzinski JJ, Rosenthal EL, Warram JM, Pinchuk AN, et al. Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol. 2017;14(6):347–64. https://doi.org/10.1038/nrclinonc.2016.212.
Liu M, Zheng S, Zhang X, Guo H, Shi X, Kang X, et al. Cerenkov luminescence imaging on evaluation of early response to chemotherapy of drug-resistant gastric cancer. Nanomedicine: Nanotechnol Biol Med. 2018;14(1):205–13. https://doi.org/10.1016/j.nano.2017.10.001.
Hu Z, Qu Y, Wang K, Zhang X, Zha J, Song T, et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat Commun. 2015;6:7560. https://doi.org/10.1038/ncomms8560.
Lauwerends LJ, van Driel PBAA, de Jong RJB, Hardillo JAU, Koljenovic S, Puppels G, et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021;22(5):e186–95. https://doi.org/10.1016/S1470-2045(20)30600-8.
Miller SE, Tummers WS, Teraphongphom N, van den Berg NS, Hasan A, Ertsey RD, et al. First-in-human intraoperative near-infrared fluorescence imaging of glioblastoma using cetuximab-IRDye800. J Neurooncol. 2018;139(1):135–43. https://doi.org/10.1007/s11060-018-2854-0.
Li D, Zhang J, Chi C, Xiao X, Wang J, Lang L, et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics. 2018;8(9):2508–20. https://doi.org/10.7150/thno.25599.
Elechalawar CK, Bhattacharya D, Ahmed MT, Gora H, Sridharan K, Chaturbedy P, et al. Dual targeting of folate receptor-expressing glioma tumor-associated macrophages and epithelial cells in the brain using a carbon nanosphere–cationic folate nanoconjugate. Nanoscale Adv. 2019;1(9):3555–67. https://doi.org/10.1039/c9na00056a.
Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol. 2015;26(10):2034–43. https://doi.org/10.1093/annonc/mdv250.
McCord E, Pawar S, Koneru T, Tatiparti K, Sau S, Iyer AK. Folate receptors’ expression in gliomas may possess potential nanoparticle-based drug delivery opportunities. ACS Omega. 2021;6(6):4111–8. https://doi.org/10.1021/acsomega.0c05500.
Licciardi M, Scialabba C, Cavallaro G, Claudio S, Elvira F, Gaetano G. Cell uptake enhancement of folate targeted polymer coated magnetic nanoparticles. J Biomed Nanotechnol. 2013;9(6):949–64. https://doi.org/10.1166/jbn.2013.1606.
Kennedy MD, Jallad KN, Thompson DH, Ben-Amotz D, Low PS. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J Biomed Opt. 2003;8(4):636–41. https://doi.org/10.1117/1.1609453.
Van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17(10):1315–9. https://doi.org/10.1038/nm.2472.
Tummers QRJG, Hoogstins CES, Gaarenstroom KN, de Kroon CD, van Poelgeest MIE, Vuyk J, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016;7(22):32144–55. https://doi.org/10.18632/oncotarget.8282.
Chen H, Shou K, Chen S, Qu C, Wang Z, Jiang L, et al. Smart self-assembly amphiphilic cyclopeptide-dye for near-infrared window-II imaging. Adv Mater. 2021;33(16):2006902. https://doi.org/10.1002/adma.202006902
Chang B, Li D, Ren Y, Qu C, Shi X, Liu R, et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat Biomed Eng. 2022;6(5):629–39. https://doi.org/10.1038/s41551-021-00773-2
Yang J, He S, Hu Z, Zhang Z, Cao C, Cheng Z, et al. In vivo multifunctional fluorescence imaging using liposome-coated lanthanide nanoparticles in near-infrared-II/IIa/IIb windows. Nano Today. 2021;38:101120. https://doi.org/10.1016/j.nantod.2021.101120.
Carr JA, Franke D, Caram JR, Perkinson CF, Saif M, Askoxylakis V, et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc Natl Acad Sci. 2018;115(17):4465–70. https://doi.org/10.1073/pnas.1718917115.
Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat Photonics. 2014;8(9):723–30. https://doi.org/10.1038/nphoton.2014.166.
Carr JA, Valdez TA, Bruns OT, Bawendi MG. Using the shortwave infrared to image middle ear pathologies. Proc Natl Acad Sci. 2016;113(36):9989–94. https://doi.org/10.1073/pnas.1610529113.
Hu Z, Chen WH, Tian J, Cheng Z. NIRF nanoprobes for cancer molecular imaging: approaching clinic. Trends Mol Med. 2020;26(5):469–82. https://doi.org/10.1016/j.molmed.2020.02.003.
Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng. 2020;4(3):259–71. https://doi.org/10.1038/s41551-019-0494-0.
Shi X, Zhang Z, Zhang Z, Cao C, Cheng Z, Hu Z, et al. Near-infrared window II fluorescence image-guided surgery of high-grade gliomas prolongs the progression-free survival of patients. IEEE Trans Biomed Eng. 2022;69:1889–1900. https://doi.org/10.1109/TBME.2021.3130195
Cao C, Jin Z, Shi X, Zhang Z, Xiao A, Yang J, et al. First clinical investigation of near-infrared window IIa/IIb fluorescence imaging for precise surgical resection of gliomas. IEEE Trans Biomed Eng. 2022. https://doi.org/10.1109/TBME.2022.3143859
Cao C, Deng S, Wang B, Shi X, Ge L, Qiu M, et al. Intraoperative near-Infrared II window fluorescence imaging-assisted nephron-sparing surgery for complete resection of cystic renal masses. Clin Transl Med. 2021;11(10):e604. https://doi.org/10.1002/ctm2.604
Nagy A, Szoke B, Schally AV. Selective coupling of methotrexate to peptide hormone carriers through a gamma-carboxamide linkage of its glutamic acid moiety: benzotriazol-1-yloxytris (dimethylamino) phosphonium hexafluorophosphate activation in salt coupling. Proc Natl Acad Sci. 1993;90(13):6373–6. https://doi.org/10.1073/pnas.90.13.6373.
Xu L, Bai Q, Zhang X, Yang H. Folate-mediated chemotherapy and diagnostics: an updated review and outlook. J Control Release. 2017;252:73–82. https://doi.org/10.1016/j.jconrel.2017.02.023.
Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discovery. 2015;14(3):203–19. https://doi.org/10.1038/nrd4519.
Guo H, Miao Y. Cu-64-labeled lactam bridge-cyclized α-MSH peptides for PET imaging of melanoma. Mol Pharm. 2012;9(8):2322–30. https://doi.org/10.1021/mp300246j.
Qiao Z, Xu J, Gonzalez R, Miao Y. Novel 64Cu-labeled NOTA-conjugated lactam-cyclized alpha-melanocyte-stimulating hormone peptides with enhanced tumor to kidney uptake ratios. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00211.
Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, et al. [64Cu-NOTA-8-Aoc-BBN (7–14) NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci. 2007;104(30):12462–7. https://doi.org/10.1073/pnas.0705347104.
Ait-Mohand S, Fournier P, Dumulon-Perreault V, Kiefer GE, Jurek P, Ferreira CL, et al. Evaluation of 64Cu-labeled bifunctional chelate–bombesin conjugates. Bioconjug Chem. 2011;22(8):1729–35. https://doi.org/10.1021/bc2002665.
Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47(6):1465–74. https://doi.org/10.1021/jm030383m.
Predina JD, Newton AD, Keating J, Barbosa EM Jr, Okusanya O, Xia L, et al. Intraoperative molecular imaging combined with positron emission tomography improves surgical management of peripheral malignant pulmonary nodules. Ann Surg. 2017;266(3):479–88. https://doi.org/10.1097/SLA.0000000000002382.
Pierce JT, Cho SS, Nag S, Zeh R, Jeon J, Holt D, et al. Folate receptor overexpression in human and canine meningiomas—immunohistochemistry and case report of intraoperative molecular imaging. Neurosurgery. 2019;85(3):359–68. https://doi.org/10.1093/neuros/nyy356.
Hoogstins CES, Tummers QRJG, Gaarenstroom KN, de Kroon CD, Trimbos JBMZ, Bosse T, et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22(12):2929–38. https://doi.org/10.1158/1078-0432.CCR-15-2640.
Shi J, Liu TWB, Chen J, Green D, Jaffray D, Wilson BC, et al. Transforming a targeted porphyrin theranostic agent into a PET imaging probe for cancer. Theranostics. 2011;1:363. https://doi.org/10.7150/thno/v01p0363.
Vaitilingam B, Chelvam V, Kularatne SA, Poh S, Ayala-Lopez W, Low PS. A folate receptor-α–specific ligand that targets cancer tissue and not sites of inflammation. J Nucl Med. 2012;53(7):1127–34. https://doi.org/10.2967/jnumed.111.099390.
Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z, et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat Biomed Eng. 2016;1(1):1–8. https://doi.org/10.1038/s41551-016-0006.
Voskuil FJ, Steinkamp PJ, Zhao T, van der Vegt B, Koller M, Doff JJ, et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat Commun. 2020;11(1):1–10. https://doi.org/10.1038/s41467-020-16814-4.
Guo H, Yu J, Hu Z, Yi H, Hou Y, He X. A hybrid clustering algorithm for multiple-source resolving in bioluminescence tomography. J Biophotonics. 2018;11(4):e201700056. https://doi.org/10.1002/jbio.201700056
Zhang Q, Zhao H, Chen D, Qu X, Chen X, He X, Li W, Hu Z, Liu J, Liang J, Tian J. Source sparsity based primal-dual interior-point method for three-dimensional bioluminescence tomography. Opt Commun. 2011;284(24):5871–6. https://doi.org/10.1016/j.optcom.2011.07.071.
Guo H, Hu Z, He X, Zhang X, Liu M, Zhang Z, et al. Non-convex sparse regularization approach framework for high multiple-source resolution in Cerenkov luminescence tomography. Opt Express. 2017;25(23):28068–85. https://doi.org/10.1364/OE.25.028068
Hu Z, Liang J, Yang W, Fan W, Li C, Ma X, et al. Experimental Cerenkov luminescence tomography of the mouse model with SPECT imaging validation. Opt Express. 2010;18(24):24441–50. https://doi.org/10.1364/OE.18.024441
Qin C, Zhong J, Hu Z, Yang X, Tian J. Recent advances in Cerenkov luminescence and tomography imaging. IEEE J Sel Top Quant Electron. 2011;18(3):1084–93. https://doi.org/10.1109/JSTQE.2011.2161757
Acknowledgements
The authors would like to acknowledge the instrumental and technical support of the multi-modal biomedical imaging experimental platform, Institute of Automation, Chinese Academy of Sciences. The authors thank Prof. Hua Zhu from the Beijing Cancer Hospital for their efforts in the use of 64Cu and radiolabeling of the probe.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFA0205200), National Natural Science Foundation of China (NSFC) (62027901, 81930053, 92059207, 81227901, 81701754), Beijing Natural Science Foundation (JQ19027), CAS Youth Interdisciplinary Team (JCTD-2021–08), the Strategic Priority Research Program (A) of Chinese Academy of Sciences (XDA16021200), and the Zhuhai High-level Health Personnel Team Project (Zhuhai HLHPTP201703).
Author information
Authors and Affiliations
Contributions
Pengfei Xu synthesized the probe. Xiaojing Shi performed the experiments. Xiaojing Shi and Pengfei Xu analyzed the data and wrote the manuscript. Zhenhua Hu, Jie Tian, and Zhen Cheng supervised the designation of the experiments. Caiguang Cao assisted with data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study only involved small animals. All the experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Institute of Automation, Chinese Academy of Sciences.
Consent for publication
All the co-authors approved the manuscript and agreed with submission to your esteemed journal. No person’s data are involved in this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, X., Xu, P., Cao, C. et al. PET/NIR-II fluorescence imaging and image-guided surgery of glioblastoma using a folate receptor α-targeted dual-modal nanoprobe. Eur J Nucl Med Mol Imaging 49, 4325–4337 (2022). https://doi.org/10.1007/s00259-022-05890-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05890-x