Abstract
Purpose
Nowadays, it is necessary to explore effective biomarkers associated with tumor immune microenvironment (TIME) noninvasively. Here, we investigated whether the metabolic parameter from preoperative 2-[18F]FDG PET/CT could provide information related to TIME in patients with clear cell renal cell carcinoma (ccRCC).
Methods
Ninety patients with newly diagnosed ccRCC who underwent 2-[18F]FDG PET/CT prior to surgery were retrospectively reviewed. The immunological features included tumor-infiltrating lymphocytes (TILs) density, programmed death-ligand 1 (PD-L1) expression, and tumor immune microenvironment types (TIMTs). TIMTs were classified as TIMT I (positive PD-L1 and high TILs), TIMT II (negative PD-L1 and low TILs), TIMT III (positive PD-L1 and low TILs), and TIMT IV (negative PD-L1 and high TILs). The relationship between maximum standardized uptake value (SUVmax) in the primary lesion from 2-[18F]FDG PET/CT and immunological features was analyzed. Cox proportional hazards analyses were performed to identify the prognostic factors for disease-free survival (DFS) after nephrectomy.
Results
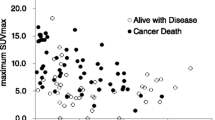
Tumors with high TILs infiltration showed remarkable correlation with elevated SUVmax and aggressive clinicopathological characteristics, such as high World Health Organization/International Society of Urological Pathology (WHO/ISUP) grade. PD-L1 expression on tumor cells was positively associated with WHO/ISUP grade and negatively correlated with body mass index (BMI). However, no correlation was observed between SUVmax and PD-L1 expression, regardless of its spatial tissue distribution. SUVmax of TIMT I and IV was higher than that of TIMT II, but there was remarkable difference merely between TIMT II and IV. In multivariate analysis, SUVmax (P = 0.022, HR 3.120, 95% CI 1.175–8.284) and WHO/ISUP grade (P = 0.046, HR 2.613, 95% CI 1.017–6.710) were the significant prognostic factors for DFS. Six cases (16.2%) with normal SUVmax showed disease progression, while 25 cases (71.4%) with elevated SUVmax experienced disease progression. Conversely, the immunological features held no prognostic value.
Conclusions
Our findings demonstrated that 2-[18F]FDG PET/CT could provide metabolic information of TIME for ccRCC patients and develop image-guided therapeutic strategies accordingly. Patients with elevated preoperative SUVmax should be seriously considered, and perioperative immunotherapy might be beneficial for them.







Similar content being viewed by others
Availability of data and material
The data used in the current study is available from the corresponding authors on reasonable request.
Code availability
Not applicable.
References
Motzer RJ, Jonasch E, Michaelson MD, Nandagopal L, Gore JL, George S, et al. NCCN guidelines insights: kidney cancer, version 2.2020. J Natl Compr Cancer Netw. 2019;17:1278–85. https://doi.org/10.6004/jnccn.2019.0054.
Beckermann KE, Hongo R, Ye X, Young K, Carbonell K, Healey DCC, et al. CD28 costimulation drives tumor-infiltrating T cell glycolysis to promote inflammation. JCI Insight. 2020;5. https://doi.org/10.1172/jci.insight.138729.
Zhu J, Armstrong AJ, Friedlander TW, Kim W, Pal SK, George DJ, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Immunother Cancer. 2018;6:4. https://doi.org/10.1186/s40425-018-0314-1.
Lavacchi D, Pellegrini E, Palmieri VE, Doni L, Mela MM, Di Maida F, et al. Immune checkpoint inhibitors in the treatment of renal cancer: current state and future perspective. Int J Mol Sci. 2020;21:4691. https://doi.org/10.3390/ijms21134691.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. https://doi.org/10.1038/nature14011.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. https://doi.org/10.1126/scitranslmed.3003689.
Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. https://doi.org/10.1158/0008-5472.Can-15-0255.
Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269:471–8. https://doi.org/10.1097/sla.0000000000002616.
Chen L, Cao MF, Zhang X, Dang WQ, Xiao JF, Liu Q, et al. The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Med. 2019;8:7207–18. https://doi.org/10.1002/cam4.2580.
Hamada T, Soong TR, Masugi Y, Kosumi K, Nowak JA, da Silva A, et al. TIME (Tumor Immunity in the MicroEnvironment) classification based on tumor CD274 (PD-L1) expression status and tumor-infiltrating lymphocytes in colorectal carcinomas. Oncoimmunology. 2018;7:e1442999. https://doi.org/10.1080/2162402x.2018.1442999.
Ock CY, Keam B, Kim S, Lee JS, Kim M, Kim TM, et al. Pan-cancer immunogenomic perspective on the tumor microenvironment based on PD-L1 and CD8 T-cell infiltration. Clin Cancer Res. 2016;22:2261–70. https://doi.org/10.1158/1078-0432.Ccr-15-2834.
Li F, Simon MC. Cancer cells don't live alone: metabolic communication within tumor microenvironments. Dev Cell. 2020;54:183–95. https://doi.org/10.1016/j.devcel.2020.06.018.
Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021;3:21–32. https://doi.org/10.1038/s42255-020-00317-z.
Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516–31. https://doi.org/10.1038/s41568-020-0273-y.
Wang Y, Zhao N, Wu Z, Pan N, Shen X, Liu T, et al. New insight on the correlation of metabolic status on (18)F-FDG PET/CT with immune marker expression in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2020;47:1127–36. https://doi.org/10.1007/s00259-019-04500-7.
Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging. 2016;43:1954–61. https://doi.org/10.1007/s00259-016-3425-2.
Jiang H, Zhang R, Jiang H, Zhang M, Guo W, Zhang J, et al. Retrospective analysis of the prognostic value of PD-L1 expression and (18)F-FDG PET/CT metabolic parameters in colorectal cancer. J Cancer. 2020;11:2864–73. https://doi.org/10.7150/jca.38689.
Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and (18)F-FDG uptake in bladder cancer. Eur J Nucl Med Mol Imaging. 2019;46:848–54. https://doi.org/10.1007/s00259-018-4208-8.
Zhao Y, Wu C, Li W, Chen X, Li Z, Liao X, et al. 2-[(18)F]FDG PET/CT parameters associated with WHO/ISUP grade in clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2021;48:570–9. https://doi.org/10.1007/s00259-020-04996-4.
Wu C, Cui Y, Zhao Y, Chen X, Liao X, Di L, et al. Elevated tumor-to-liver standardized uptake value ratio (TLR) from preoperative (18)F-FDG PET/CT predicts poor prognosis of patients with clear cell renal cell carcinoma after nephrectomy. Eur J Radiol. 2020;131:109218. https://doi.org/10.1016/j.ejrad.2020.109218.
Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol. 2016;34:3655–63. https://doi.org/10.1200/jco.2016.66.7311.
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immuno-oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–35. https://doi.org/10.1097/pap.0000000000000161.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. https://doi.org/10.1056/NEJMoa1712126.
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–15. https://doi.org/10.1016/s0140-6736(19)30723-8.
Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharmacologic intervention, part 1: the role of (18)F-FDG PET/CT. J Nucl Med. 2020;61:943–50. https://doi.org/10.2967/jnumed.119.234278.
Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35:965–73. https://doi.org/10.1002/bies.201300084.
Hirakata T, Fujii T, Kurozumi S, Katayama A, Honda C, Yanai K, et al. FDG uptake reflects breast cancer immunological features: the PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res Treat. 2020;181:331–8. https://doi.org/10.1007/s10549-020-05619-0.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. https://doi.org/10.1038/s41573-018-0007-y.
Lee KS, Yun S, Lee K, Moon S, Choe G. Clinicopathological implications of the expression of vascular endothelial growth factor and programmed death ligand 1 in clear-cell renal cell carcinoma. Hum Pathol. 2020;99:88–97. https://doi.org/10.1016/j.humpath.2020.03.013.
Xiao WJ, Xu FJ, Zhang X, Zhou SX, Zhang HL, Dai B, et al. The prognostic value of programmed death-ligand 1 in a Chinese cohort with clear cell renal cell carcinoma. Front Oncol. 2019;9:879. https://doi.org/10.3389/fonc.2019.00879.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. https://doi.org/10.1038/nrc3239.
Davis D, Tretiakova MS, Kizzar C, Woltjer R, Krajbich V, Tykodi SS, et al. Abundant CD8+ tumor infiltrating lymphocytes and beta-2-microglobulin are associated with better outcome and response to interleukin-2 therapy in advanced stage clear cell renal cell carcinoma. Ann Diagn Pathol. 2020;47:151537. https://doi.org/10.1016/j.anndiagpath.2020.151537.
Siddiqui SA, Frigola X, Bonne-Annee S, Mercader M, Kuntz SM, Krambeck AE, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–81. https://doi.org/10.1158/1078-0432.Ccr-06-2139.
Yao J, Xi W, Zhu Y, Wang H, Hu X, Guo J. Checkpoint molecule PD-1-assisted CD8(+) T lymphocyte count in tumor microenvironment predicts overall survival of patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Cancer Manag Res. 2018;10:3419–31. https://doi.org/10.2147/cmar.S172039.
Ning XH, Gong YQ, He SM, Li T, Wang JY, Peng SH, et al. Higher programmed cell death 1 ligand 1 (PD-L1) mRNA level in clear cell renal cell carcinomas is associated with a favorable outcome due to the active immune responses in tumor tissues. Oncotarget. 2017;8:3355–63. https://doi.org/10.18632/oncotarget.13765.
Chipollini J, da Costa WH, Werneck da Cunha I, de Almeida EPF, Guilherme OSP, Azizi M, et al. Prognostic value of PD-L1 expression for surgically treated localized renal cell carcinoma: implications for risk stratification and adjuvant therapies. Ther Adv Urol. 2019;11:1756287219882600. https://doi.org/10.1177/1756287219882600.
Wang Q, Lou W, Di W, Wu X. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol. 2017;52:7–14. https://doi.org/10.1016/j.intimp.2017.08.017.
Ringel AE, Drijvers JM, Baker GJ, Catozzi A, García-Cañaveras JC, Gassaway BM, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–66.e26. https://doi.org/10.1016/j.cell.2020.11.009.
Chen H, Wang D, Zhong Q, Tao Y, Zhou Y, Shi Y. Pretreatment body mass index and clinical outcomes in cancer patients following immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2020;69:2413–24. https://doi.org/10.1007/s00262-020-02680-y.
Acknowledgments
We express sincere gratitude to Dr. Shuai Hu for his instructive advice on the methods of immunohistochemistry and Yiyi Ma for her help in the immunohistochemistry staining procedure.
Funding
This study was supported by grants from the Clinical Medicine Plus X-Young Scholars Project of Peking University, the Fundamental Research Funds for the Central Universities (PKU2018LCXQ012, PKU2019LCXQ021), the Beijing Science and Technology Project (Z181100001618017), and the Youth Clinical Research Project of Peking University First Hospital (2018CR14).
Author information
Authors and Affiliations
Contributions
Conceptualization: Meng Liu and Yan Fan
Methodology: Meng Liu, Yan Xiong, Yanqing Gong, Qun He, and Jianhua Zhang
Formal analysis and investigation: Caixia Wu, Yonggang Cui, Jumei Liu, Linlin Ma, Yanyan Zhao, Xi Zhang, and Silu Chen
Writing-original draft preparation: Caixia Wu, Yonggang Cui, and Jumei Liu
Writing-review and editing: Meng Liu and Yan Fan
Funding acquisition: Meng Liu and Yan Fan
Supervision: Meng Liu and Yan Fan
All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board (IRB) of Peking University First Hospital approved this retrospective study and waived the need for written informed consent.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary.
Rights and permissions
About this article
Cite this article
Wu, C., Cui, Y., Liu, J. et al. Noninvasive evaluation of tumor immune microenvironment in patients with clear cell renal cell carcinoma using metabolic parameter from preoperative 2-[18F]FDG PET/CT. Eur J Nucl Med Mol Imaging 48, 4054–4066 (2021). https://doi.org/10.1007/s00259-021-05399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05399-9