Abstract
Background
Amivantamab is a novel bispecific antibody that simultaneously targets the epidermal growth factor receptor (EGFR) and the hepatocyte growth factor receptor (HGFR/c-MET) that are overexpressed in several types of cancer including triple-negative breast cancer (TNBC). Targeting both receptors simultaneously can overcome resistance to mono-targeted therapy. The purpose of this study is to develop 89Zr-labeled amivantamab as a potential companion diagnostic imaging agent to amivantamab therapy using various preclinical models of TNBC for evaluation.
Methods
Amivantamab was conjugated to desferrioxamine (DFO) and radiolabeled with 89Zr to obtain [89Zr]ZrDFO-amivantamab. Binding of the bispecific [89Zr]ZrDFO-amivantamab as well as its mono-specific “single-arm” antibody controls were determined in vitro and in vivo. Biodistribution studies of [89Zr]ZrDFO-amivantamab were performed in MDA-MB-468 xenografts to determine the optimal imaging time point. PET/CT imaging with [89Zr]ZrDFO-amivantamab or its isotype control was performed in a panel of TNBC xenografts with varying levels of EGFR and c-MET expression.
Results
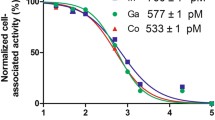
[89Zr]ZrDFO-amivantamab was synthesized with a specific activity of 148 MBq/mg and radiochemical yield of ≥ 95%. Radioligand binding studies and western blot confirmed the order of EGFR and c-MET expression levels: HCC827 lung cancer cell (positive control) > MDA-MB-468 > MDA-MB-231 > MDA-MB-453. [89Zr]ZrDFO-amivantamab demonstrated bispecific binding in cell lines co-expressed with EGFR and c-MET. PET/CT imaging with [89Zr]ZrDFO-amivantamab in TNBC xenografted mice showed standard uptake value (SUVmean) of 6.0 ± 1.1 in MDA-MB-468, 4.2 ± 1.4 in MDA-MB-231, and 1.5 ± 1.4 in MDA-MB-453 tumors, which are consistent with their receptors’ expression levels on the cell surface.
Conclusion
We have successfully prepared a radiolabeled bispecific antibody, [89Zr]ZrDFO-amivantamab, and evaluated its pharmacologic and imaging properties in comparison with its single-arm antibodies and non-specific isotype controls. [89Zr]ZrDFO-amivantamab demonstrated the greatest uptake in tumors co-expressing EGFR and c-MET.












Similar content being viewed by others
References
Chae YK, Gagliato Dde M, Pai SG, Carneiro B, Mohindra N, Giles FJ, et al. The association between EGFR and cMET expression and phosphorylation and its prognostic implication in patients with breast cancer. PLoS One. 2016;11(4). https://doi.org/10.1371/journal.pone.0152585.
Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. https://doi.org/10.4103/1477-3163.44372.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. https://doi.org/10.1172/JCI45014.
Linklater ES, Tovar EA, Essenburg CJ, Turner L, Madaj Z, Winn ME, et al. Targeting MET and EGFR crosstalk signaling in triple-negative breast cancers. Oncotarget. 432016:69903–15.
Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30(21):2615–23. https://doi.org/10.1200/JCO.2010.34.5579.
Dieras V, Campone M, Yardley DA, Romieu G, Valero V, Isakoff SJ, et al. Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer. Ann Oncol. 2015;26(9):1904–10. https://doi.org/10.1093/annonc/mdv263.
Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. https://doi.org/10.1038/s41573-019-0028-1.
Study of JNJ-61186372, a human bispecific EGFR and cMet antibody, in participants with advanced non-small cell lung cancer - Tabular View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT02609776 (2020). Accessed.
Moores SL, Chiu ML, Bushey BS, Chevalier K, Luistro L, Dorn K, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76(13):3942–53. https://doi.org/10.1158/0008-5472.can-15-2833.
Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–47. https://doi.org/10.1016/j.drudis.2015.02.008.
Grugan KD, Dorn K, Jarantow SW, Bushey BS, Pardinas JR, Laquerre S, et al. Fc-mediated activity of EGFR x c-Met bispecific antibody JNJ-61186372 enhanced killing of lung cancer cells. MAbs. 2017;9(1):114–26. https://doi.org/10.1080/19420862.2016.1249079.
Cho BC, Lee J-S, Han J-Y, Cho EK, Haura E, Lee KH, et al. 1497PJNJ-61186372 (JNJ-372), an EGFR-cMET bispecific antibody, in advanced non-small cell lung cancer (NSCLC): an update on phase I results. Annals of Oncology. 2018;29(suppl_8). https://doi.org/10.1093/annonc/mdy292.118.
de Matos LL, Trufelli DC, de Matos MGL, da Silva Pinhal MA. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights. 2010;5:9–20.
Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. 2012;23 Suppl 6:vi19–22. https://doi.org/10.1093/annonc/mds190.
Pozner-Moulis S, Cregger M, Camp RL, Rimm DL. Antibody validation by quantitative analysis of protein expression using expression of Met in breast cancer as a model. Lab Investig. 2007;87(3):251–60. https://doi.org/10.1038/labinvest.3700515.
Knudsen BS, Zhao P, Resau J, Cottingham S, Gherardi E, Xu E, et al. A novel multipurpose monoclonal antibody for evaluating human c-Met expression in preclinical and clinical settings. Appl Immunohistochem Mol Morphol. 2009;17(1):57–67. https://doi.org/10.1097/PAI.0b013e3181816ae2.
McKnight BN, Viola-Villegas NT. 89Zr-ImmunoPET companion diagnostics and their impact in clinical drug development. J Labelled Comp Radiopharm. 2018;61(9):727–38. https://doi.org/10.1002/jlcr.3605.
Marquez-Nostra BV, Viola N. The Radiopharmaceutical Chemistry of Zirconium-89. In: Jason S. Lewis ADW, Brian M. Zeglis, ed. Radiopharmaceutical Chemistry: Springer Charm. 2019;371–390.
McKnight BN, Kuda-Wedagedara ANW, Sevak KK, Abdel-Atti D, Wiesend WN, Ku A, et al. Imaging EGFR and HER3 through 89 Zr-labeled MEHD7945A (Duligotuzumab). Sci Rep. 2018;8(1):1–13. https://doi.org/10.1038/s41598-018-27454-6.
Moek KL, Waaijer SJH, Kok IC, Suurs FV, Brouwers AH, Oordt CWM-vdHv, et al. 89Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, Heterogeneous Tumor Uptake. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.CCR-18-2918.
Kim EM, Jeong MH, Kim DW, Jeong HJ, Lim ST, Sohn MH. Iodine 125-labeled mesenchymal-epithelial transition factor binding peptide-click-cRGDyk heterodimer for glioma imaging. Cancer Sci. 2011;102(8):1516–21. https://doi.org/10.1111/j.1349-7006.2011.01983.x.
Robinson MK, Hodge KM, Horak E, Sundberg ÅL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99(9):1415–25. https://doi.org/10.1038/sj.bjc.6604700.
Luo H, Hong H, Yang SP, Cai W. Design and applications of bispecific heterodimers: molecular imaging and beyond. Mol Pharm. 2014;11(6):1750–61. https://doi.org/10.1021/mp500115x.
Marquez-Nostra BV, Lee S, Laforest R, Vitale L, Nie X, Hyrc K, et al. Preclinical PET imaging of glycoprotein non-metastatic melanoma B in triple negative breast cancer: feasibility of an antibody-based companion diagnostic agent. Oncotarget. 2017;8(61):104303–14. https://doi.org/10.18632/oncotarget.22228.
Encarnação JC, Barta P, Fornstedt T, Andersson K. Impact of assay temperature on antibody binding characteristics in living cells: a case study. Biomed Rep. 2017;7(5):400–6. https://doi.org/10.3892/br.2017.982.
Abou D, Ku T, Smith-Jones P. In vivo biodistribution and accumulation of 89Zr in mice. Nucl Med Biol. 2011;38(5):675–81. https://doi.org/10.1016/j.nucmedbio.2010.12.011.
Acknowledgments
We thank the Yale PET Center for the use of the small animal PET/CT scanner, Washington University in St. Louis and 3D Imaging LLC for the production of 89Zr-oxalate, and Yale Pathology Translational Services for the immunohistochemical analyses.
Funding
This research was funded by the Lion Hearts. Mr. Lee, Dr. Cavaliere and Dr. Marquez-Nostra are supported in part by the NCI R00 CA201601.
Author information
Authors and Affiliations
Contributions
AC, SS, SM, and BMN contributed to the study conception and design. Material preparation and data collection were performed by AC, SS, SL, JB, and ZL. All the authors contributed to the data analysis. The manuscript was written by AC, SL, and JB and edited by BMN, SS, and YH. All the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Sheri Moores is an employee of Janssen Pharmaceuticals. The authors have declared that no competing interest exists.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Electronic supplementary material
ESM 1
(DOCX 1973 kb)
Rights and permissions
About this article
Cite this article
Cavaliere, A., Sun, S., Lee, S. et al. Development of [89Zr]ZrDFO-amivantamab bispecific to EGFR and c-MET for PET imaging of triple-negative breast cancer. Eur J Nucl Med Mol Imaging 48, 383–394 (2021). https://doi.org/10.1007/s00259-020-04978-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04978-6