Abstract
Purpose
A major challenge for accurate quantitative SPECT imaging of some radionuclides is the inadequacy of simple energy window-based scatter estimation methods, widely available on clinic systems. A deep learning approach for SPECT/CT scatter estimation is investigated as an alternative to computationally expensive Monte Carlo (MC) methods for challenging SPECT radionuclides, such as 90Y.
Methods
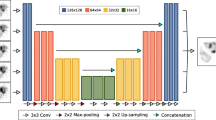
A deep convolutional neural network (DCNN) was trained to separately estimate each scatter projection from the measured 90Y bremsstrahlung SPECT emission projection and CT attenuation projection that form the network inputs. The 13-layer deep architecture consisted of separate paths for the emission and attenuation projection that are concatenated before the final convolution steps. The training label consisted of MC-generated “true” scatter projections in phantoms (MC is needed only for training) with the mean square difference relative to the model output serving as the loss function. The test data set included a simulated sphere phantom with a lung insert, measurements of a liver phantom, and patients after 90Y radioembolization. OS-EM SPECT reconstruction without scatter correction (NO-SC), with the true scatter (TRUE-SC) (available for simulated data only), with the DCNN estimated scatter (DCNN-SC), and with a previously developed MC scatter model (MC-SC) were compared, including with 90Y PET when available.
Results
The contrast recovery (CR) vs. noise and lung insert residual error vs. noise curves for images reconstructed with DCNN-SC and MC-SC estimates were similar. At the same noise level of 10% (across multiple realizations), the average sphere CR was 24%, 52%, 55%, and 67% for NO-SC, MC-SC, DCNN-SC, and TRUE-SC, respectively. For the liver phantom, the average CR for liver inserts were 32%, 73%, and 65% for NO-SC, MC-SC, and DCNN-SC, respectively while the corresponding values for average contrast-to-noise ratio (visibility index) in low-concentration extra-hepatic inserts were 2, 19, and 61, respectively. In patients, there was high concordance between lesion-to-liver uptake ratios for SPECT reconstruction with DCNN-SC (median 4.8, range 0.02–13.8) compared with MC-SC (median 4.0, range 0.13–12.1; CCC = 0.98) and with 90Y PET (median 4.9, range 0.02–11.2; CCC = 0.96) while the concordance with NO-SC was poor (median 2.8, range 0.3–7.2; CCC = 0.59). The trained DCNN took ~ 40 s (using a single i5 processor on a desktop computer) to generate the scatter estimates for all 128 views in a patient scan, compared to ~ 80 min for the MC scatter model using 12 processors.
Conclusions
For diverse 90Y test data that included patient studies, we demonstrated comparable performance between images reconstructed with deep learning and MC-based scatter estimates using metrics relevant for dosimetry and for safety. This approach that can be generalized to other radionuclides by changing the training data is well suited for real-time clinical use because of the high speed, orders of magnitude faster than MC, while maintaining high accuracy.







Similar content being viewed by others
Data availability
Python code for the DCNN of Fig. 2 and phantom training and test data are available at https://github.com/haoweix/spect-scatter-deep-learning. Select 90Y SPECT/CT patient test data sets (anonymized) are available at the University of Michigan Library Deep Blue repository: https://doi.org/10.7302/v07v-z854.
References
Zeintl J, Vija AH, Yahil A, Hornegger J, Kuwert T. Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med. 2010;51:921–8.
Elschot M, Lam MG, van den Bosch MA, Viergever MA, de Jong HW. Quantitative Monte Carlo based 90Y SPECT reconstruction. J Nucl Med. 2013;54:1557–63.
Dewaraja YK, et al. Improved quantitative 90Y bremsstrahlung SPECT/CT reconstruction with Monte Carlo scatter modeling. Med Phys. 2017;44:6364–76.
Greenspan BH, van Ginneken B, Summers RM. Guest editorial—deep learning in medical imaging: overview and future promise of an exciting new techniques. IEEE Trans Med Imag. 2016;35:1153–9.
Ravishankar S, Ye JC, Fessler JA. Image reconstruction: from sparsity to data-adaptive methods and machine learning. Proc IEEE. 2020;108:86–10.
Veit-Haibach P, Buvat I, Herrmann K. EJNMMI supplement: bringing AI and radiomics to nuclear medicine. Eur J Nucl Med Mol Imaging. 2019;46:2627–9.
Uribe CF, Mathotaarachchi S, Gaudet V, Smith KC, Rosa-Neto P, Bénard F, et al. Machine learning in nuclear medicine: part 1-introduction. J Nucl Med. 2019;60:451–8.
Zaharchuk G. Next generation research applications for hybrid PET/MR and PET/CT imaging using deep learning. Eur J Nucl Med Mol Imaging. 2019;46:2700–7.
Gong K, Berg E, Cherry SR, Qi J. Machine learning in PET: from photon detection to quantitative image reconstruction. Proc IEEE. 2020;108:51–68.
Xu J, Gong E, Pauly J, Zaharchuk G. 200x low-dose PET reconstruction using deep learning, arXiv preprint arXiv:1712.04119, 2017.
Berker Y, Joscha M, Kachelries M. Deep scatter estimation in PET: fast scatter correction using a convolutional neural network. Proc IEEE Nucl Sci Symp Med Imag Conf. 2018:1–25.
Qian H, Rui X, Ahn S. Deep learning models for PET scatter estimations. Proc IEEE Nucl Sci Symp Med Imag Conf. 2017:1–5.
Yang J, Park D, Gullberg GT, Seo Y. Joint correction of attenuation and scatter in image space using deep convolutional neural networks for dedicated brain (18)F-FDG PET. Phys Med Biol. 2019;64:075019.
Häggström I, Schmidtlein CR, Campanella G, Fuchs TJ. DeepPET: a deep encoder-decoder network for directly solving the PET image reconstruction inverse problem. Med Image Anal. 2019;54:253–62.
Cui J, Gong K, Guo N, et al. PET image denoising using unsupervised deep learning. Eur J Nucl Med Mol Imaging. 2019;46:2780–9.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation . 2015. https://arxiv.org/abs/1505.04597
Dietze MMA, Branderhorst W, Kunnen B, Viergever MA, de Jong HWAM. Accelerated SPECT image reconstruction with FBP and an image enhancement convolutional neural network. EJNMMI Phys. 2019;6:14.
Minarik D, Enqvist O, Trägårdh E. Denoising of scintillation camera images using a deep convolutional neural network: a Monte Carlo simulation approach. J Nucl Med. 2020;61:298–303.
Shi L, Onofrey JA, Liu H, et al. Deep learning-based attenuation map generation for myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04746-6.
Bai J, Hashimoto J, Ogawa K, et al. Scatter correction based on an artificial neural network for 99mTc and 123I dual-isotope SPECT in myocardial and brain imaging. Ann Nucl Med. 2007;21:25–32.
Ogawa K, Nishizaki N. Accurate scatter compensation using neural networks in radionuclide imaging. IEEE Trans Nucl Sci. 1993;40:1020–5.
El Fakhri G, Moore SC, Maksud P, Aurengo A, Kijewski MF. Absolute activity quantitation in simultaneous 123I/99mTc brain SPECT. J Nucl Med. 2001;42:300–8.
Simonyan, K., Zisserman, A., 2014. Very deep convolutional networks for large-scale image recognition, 1409.1556, pp. 1–14. https://arxiv.org/abs/1409.1556v6
K. He, X. Zhang, S. Ren and J. Sun, "Deep residual learning for image recognition," 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, 2016, pp. 770–778. or https://arxiv.org/abs/1512.03385
D. P. Kingma and J. Ba, Adam: a method for stochastic optimization, 2017 https://arxiv.org/abs/1412.6980
Abadi M, Agarwal A, Barham P. TensorFlow: large-scale machine learning on heterogeneous distributed systems https://arxiv.org/abs/1603.04467
Ljungberg M. The SIMIND Monte Carlo program. In: Ljungberg M, Strand SE, King MA, editors. Monte Carlo calculation in nuclear medicine: application in diagnostic imaging. 2nd ed. Florida: Taylor & Francis; 2012.
Dewaraja YK, Fleming R, Simpson P, Ljungberg M, Wilderman S. Impact of internal bremsstrahlung on Y-90 SPECT Imaging. J Nucl Med. 2018;59(supplement 1):577.
Segars W, Sturgeon G, Mendonca S, Grimes J, Tsui BM. 4D XCAT phantom for multimodality imaging research. Med Phys. 2010;37:4902–15.
NEMA 2012 PET phantom digital reference object. Developed at the University of Washington supported by the Quantitative Imaging Biomarker Alliance. https://depts.washington.edu/petctdro/DROsuv_main.html
Rose A. Vision: human and electronic. New York: Plenum Press; 1973. p. 1–27.
Acknowledgments
We would like to thank Phantech, Madison, Wisconsin, for providing us the 3-D printed phantom insert.
Funding
This work was supported by grant R01 EB022075 awarded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), United States Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study according to University of Michigan Institutional Review Board (IRB) criteria.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence).
Electronic supplementary material
Supplementary Figure 1
Results from training and validation. (a) Convergence behavior of the MSE (average over all pixels and all training data) between DCNN estimated scatter projections and the simulated true scatter projections. (b) Profile across a typical scatter projection in the training data at epoch 100. (PPTX 51 kb)
Supplementary Table 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Xiang, H., Lim, H., Fessler, J.A. et al. A deep neural network for fast and accurate scatter estimation in quantitative SPECT/CT under challenging scatter conditions. Eur J Nucl Med Mol Imaging 47, 2956–2967 (2020). https://doi.org/10.1007/s00259-020-04840-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04840-9