Abstract
Purpose
The objective of this phase IIa, open-label, single-centre, single-arm, two-stage clinical trial was to evaluate the safety and activity of 177-lutetium DOTATATE (LuDO) molecular radiotherapy in neuroblastoma.
Methods
Children with relapsed or refractory metastatic high-risk neuroblastoma were treated with up to four courses of LuDO. The administered activity was 75 to 100 MBq kg−1 per course, spaced at 8- to 12-week intervals. Outcomes were assessed by the International Neuroblastoma Response Criteria (primary outcome), progression-free survival (PFS), and overall survival (OS).
Results
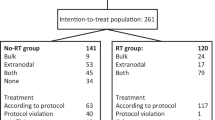
The trial recruited 21 patients; eight received the planned four courses. There was dose-limiting haematologic toxicity in one case, but no other significant haematologic or renal toxicities. None of 14 evaluable patients had an objective response at 1 month after completion of treatment (Wilson 90% CI 0.0, 0.16; and 95% CI is 0.0, 0.22). The trial did not therefore proceed to the second stage. The median PFS was 2.96 months (95% CI 1.71, 7.66), and the median OS was 13.0 months (95% CI 2.99, 21.52).
Conclusion
In the absence of any objective responses, the use of LuDO as a single agent at the dose schedule used in this study is not recommended for the treatment of neuroblastoma. There are several reasons why this treatment schedule may not have resulted in objective responses, and as other studies do show benefit, the treatment should not be regarded as being of no value. Further trials designed to overcome this schedule’s limitations are required.
Trial registration
ISRCTN98918118; URL: https://www.isrctn.com/search?q=98918118



Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- FDG:
-
Fluorodeoxyglucose
- GaDO:
-
68-Gallium DOTATATE
- GFR:
-
Glomerular filtration rate
- Gy:
-
Gray
- h:
-
Hour
- INRC:
-
International Neuroblastoma Response Criteria
- kg:
-
Kilogram
- kVp:
-
Kilovoltage peak
- l:
-
Litre
- L:
-
Levo
- LuDO:
-
177-Lutetium DOTATATE
- m:
-
Metre
- mA:
-
Milliamperes
- MBq:
-
Megabecquerel
- mIBG:
-
Meta-iodobenzylguanidine
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- PET:
-
Positron emission tomography
- PFS:
-
Progression-free survival
- PRRT:
-
Peptide receptor radionuclide therapy
- SAE:
-
Serious adverse event
- SIOPEN:
-
International Society of Paediatric Oncology European Neuroblastoma clinical research group
- SPECT:
-
Single-photon emission computed tomography
- SUVmax :
-
Maximum standardized uptake value
- UK:
-
United Kingdom
- USA:
-
United States of America
- USAN:
-
United States Adopted Name
References
Gains J, Mandeville H, Cork N, et al. Ten challenges in the management of neuroblastoma. Future Oncol. 2012;8:839–58.
Moroz V, Machin D, Faldum A, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: a report from the International Neuroblastoma Risk Group Project. Eur J Cancer. 2011;47:561–71.
Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303.
Bagatell R, Beck-Popovic M, London WB, et al. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. J Clin Oncol. 2009;27:365–70.
Cohn SL, Pearson ADJ, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–97.
Pearson AD, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–56.
Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–13.
Ladenstein R, Pötschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–14.
Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018. https://doi.org/10.1016/S1470-2045(18)30578-3.
von Allmen D, Davidoff AM, London WB, et al. Impact of extent of resection on local control and survival in patients from the COG A3973 study with high-risk neuroblastoma. J Clin Oncol. 2017;35:208–16.
Arumugam S, Manning-Cork NJ, Gains JE, et al. The evidence for external beam radiotherapy in high-risk neuroblastoma of childhood: a systematic review. Clin Oncol (R Coll Radiol). 2018. https://doi.org/10.1016/j.clon.2018.11.031.
Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–24.
Basta NO, Halliday GC, Makin G, et al. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Cancer. 2016;115:1048–57.
Wheldon TE, Livingstone A, Wilson L, et al. The radiosensitivity of human neuroblastoma cells estimated from regrowth curves of multicellular tumour spheroids. Br J Radiol. 1985;58:661–4.
Gaze MN, Boterberg T, Dieckmann K, et al. Results of a quality assurance review of external beam radiation therapy in the International Society of Paediatric Oncology (Europe) Neuroblastoma Group’s High-risk Neuroblastoma Trial: a SIOPEN study. Int J Radiat Oncol Biol Phys. 2013;85:170–4.
Gaze MN, Gains JE, Walker C, et al. Optimization of molecular radiotherapy with [131I]-meta iodobenzylguanidine for high-risk neuroblastoma. Q J Nucl Med Mol Imaging. 2013;57:66–78.
Glowniak JV, Kilty JE, Amara SG, et al. Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med. 1993;34:1140–6.
Wilson JS, Gains JE, Moroz V, et al. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50:801–15.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46:62S–6S.
Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16.
Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med Jan. 2017;376:125–35.
Strosberg J, Wolin E, Chasen B, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J Clin Oncol. 2018;36:2578–84.
Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19.
Gains JE, Sebire NJ, Moroz V, et al. Immunohistochemical evaluation of molecular radiotherapy target expression in neuroblastoma tissue. Eur J Nucl Med Mol Imaging. 2018;45:402–11.
Gains JE, Bomanji JB, Fersht NL, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. 2011;52:1041–7.
Alexander N, Vali R, Ahmadzadehfar H, et al. The role of radiolabeled DOTA-conjugated peptides for imaging and treatment of childhood neuroblastoma. Curr Radiopharm. 2018;11:14–21.
Gains JE, Walker C, Sullivan TM, et al. Radiation exposure to comforters and carers during paediatric molecular radiotherapy. Pediatr Blood Cancer. 2015;62:235–9.
Lewington V, Lambert B, Poetschger U, et al. 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting method by an international panel. Eur J Nucl Med Mol Imaging. 2017;44:234–41.
Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–43.
Buckley SE, Chittenden SJ, Saran FH, et al. Whole-body dosimetry for individualized treatment planning of 131I-MIBG radionuclide therapy for neuroblastoma. J Nucl Med. 2009;50:1518–24.
Trieu M, DuBois SG, Pon E, et al. Impact of whole-body radiation dose on response and toxicity in patients with neuroblastoma after therapy with 131 I-metaiodobenzylguanidine (MIBG). Pediatr Blood Cancer. 2016;63:436–42.
Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–81.
Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77.
Park JR, Bagatell R, Cohn SL, et al. Revisions to the International Neuroblastoma Response Criteria: a consensus statement from the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2017;35:2580–7.
Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10.
Machin D, Campbell MJ, Tan SB, et al. Sample size tables for clinical studies (ed 3). Chichester: Wiley-Blackwell; 2009.
Gaze MN, Gains JE, Bomanji JB. Current issues in molecular radiotherapy in children. In: Mansi L, Lopci E, Cuccurullo V, Chiti A, editors. Clinical nuclear medicine in pediatrics. Basel: Springer; 2016. p. 29–49.
Kong G, Hofman MS, Murray WK, et al. Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J Pediatr Hematol Oncol. 2016;38:87–96.
Moreno L, Rubie H, Varo A, et al. Outcome of children with relapsed or refractory neuroblastoma: a meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64:25–31.
Zhou MJ, Doral MY, DuBois SG, et al. Different outcomes for relapsed versus refractory neuroblastoma after therapy with (131)I-metaiodobenzylguanidine ((131)I-MIBG). Eur J Cancer. 2015;51:2465–72.
Gaze MN, Chang YC, Flux GD, et al. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm. 2005;20:195–9.
Buckley SE, Saran FH, Gaze MN, et al. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: preliminary results. Cancer Biother Radiopharm. 2007;22:105–12.
McCluskey AG, Boyd M, Gaze MN, et al. [131I]MIBG and topotecan: a rationale for combination therapy for neuroblastoma. Cancer Lett. 2005;228:221–7.
McCluskey AG, Mairs RJ, Tesson M, et al. Inhibition of poly(ADP-Ribose) polymerase enhances the toxicity of 131I-metaiodobenzylguanidine/topotecan combination therapy to cells and xenografts that express the noradrenaline transporter. J Nucl Med. 2012;53:1146–54.
Tesson M, Vasan R, Hock A, et al. An evaluation in vitro of the efficacy of nutlin-3 and topotecan in combination with 177Lu-DOTATATE for the treatment of neuroblastoma. Oncotarget. 2018;9:29082–96.
https://clinicaltrials.gov/ct2/show/NCT04023331?term=sartate&rank=2 [last accessed 30 September 2019].
Funding
This trial was funded by the Cancer Research UK (reference C17807/A14091) and Joining Against Cancer in Kids, and supported by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
Conception and design: Jennifer E. Gains, Veronica Moroz, Matthew D. Aldridge, Jamshed B. Bomanji, Keith Wheatley, Mark N. Gaze
Clinical care of patients: Jennifer E. Gains, Connie Peet, Matthew D. Aldridge, S Wan, Jamshed B. Bomanji, Mark N. Gaze
Collection and assembly of data: Connie Peet, Matthew D. Aldridge, Jennifer Laidler
Data analysis and interpretation: Jennifer E. Gains, Veronica Moroz, Simon Wan, Jamshed B. Bomanji, Matthew D. Aldridge, Keith Wheatley, Mark N. Gaze
Manuscript writing: all authors
Final approval of the manuscript: all authors
Accountable for all aspects of work: all authors
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric
Rights and permissions
About this article
Cite this article
Gains, J.E., Moroz, V., Aldridge, M.D. et al. A phase IIa trial of molecular radiotherapy with 177-lutetium DOTATATE in children with primary refractory or relapsed high-risk neuroblastoma. Eur J Nucl Med Mol Imaging 47, 2348–2357 (2020). https://doi.org/10.1007/s00259-020-04741-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04741-x