Abstract
Introduction
One of the concepts of theranostics in nuclear medicine is peptide receptor radionuclide therapy (PRRT), whereby labeled somatostatin analogs are used for imaging and treating inoperable or disseminated neuroendocrine tumors (NET).
Aim
The aim of the study was to determine the therapeutic efficacy and toxicity of tandem 90Y /177Lu-DOTATATE in patients with disseminated NET in a multicenter trial.
Materials and methods
103 patients with NET G1/G2 treated with 90Y/177Lu-DOTATATE (1:1) with amino-acid infusion for nephroprotection were included in the study.
Results
Overall survival from the disease diagnosis (OS-D) was 127.4 months and from the time of PRRT (OS-T) was 89.5 months. Progression-free survival (PFS) was 29.9 months. An analysis based on the proliferation index revealed a statistically significant impact on PFS and OS-T (PFS G1 vs G2, 59.3 vs 24.3 months; OS-T G1 vs G2, not reached vs 79.9 months). The effect of the primary disease site was also analyzed. For pancreatic vs small bowel vs large bowel, the PFS was 30.8 vs 30.3 vs 40.6 months, the OS-T was 94 vs 61.9 vs 131.2 months and OS-D was 130.4 vs 89.2 vs not reached months, respectively. The 2-year risk of progression was 42%. The probability of 2-year and 5-year overall survival was 89% and 62%, respectively. PRRT was well tolerated by all patients. One patient (1%) developed myelodysplastic syndrome. No other grade 3 and 4 hematological or renal toxicity was observed.
Conclusions
This multicenter trial showed that tandem 90Y/177Lu-DOTATATE is highly effective and safe therapy for patients with disseminated NET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term theranostics in nuclear medicine refers to combined diagnostic imaging and therapy using the same molecule. One of the most successful examples of the theranostics concept in nuclear medicine is peptide receptor radionuclide therapy (PRRT) for imaging and treatment of well differentiated neuroendocrine tumors (NET).
Neuroendocrine tumors are a heterogeneous group of neoplasms, arising from cells of the endocrine system, with various clinical behaviors. Although they may show hormonal activity (known as hormonally active tumors), a significant proportion do not produce enough hormones and/or biogenic amines to cause clinical symptoms (classed as hormonally inactive tumors) [1,2,3,4]. Although these neoplasms are considered rare, a significant increase in the incidence and detectability of NET has been noted in many epidemiological studies in recent years [2,3,4,5].
Among many options for pharmacological treatment of these tumors, long-acting somatostatin analogs which not only reduce symptoms of the disease but also have anti-proliferative effects [6, 7] remain the mainstay therapy. For patients with inoperable disease or whose disease progresses despite long-acting somatostatin analog therapy, PRRT is a reasonable second-line approach. Confirmation of somatostatin receptor overexpression by nuclear medicine imaging [1, 8] is a prerequisite for patient selection.
Initially, trials with [111In-diethylenetriaminepentaacetic acid (DTPA)0]-octreotide showed limited patient responses which were, in part, related to the physical characteristics of 111In [9, 10].
Higher response rates were obtained using the β-emitters, 90Y-DOTA0-Tyr3–octreotide (90Y-DOTATOC) and 177Lu-DOTA0-Tyr3–octreotate (177Lu-DOTATATE), which had a greater impact on tumor volume due to superior tissue penetration. Studies assessing the efficacy of 90Y-DOTATOC showed a favorable response to treatment in 10–34% of patients [11,12,13].
Due to renal excretion, the high maximum energy ([Emax] 2.27 MeV) and a long maximum particle range (10 mm) beta-particle emission by 90Y with has been shown to cause significant nephro toxicity. In contrast, 177Lu emits lower energy ([Emax] 0.497 MeV) and shorter particle range (maximum 2–4 mm) beta particles, and is an alternative to 90Y. The clinical results of [177Lu-DOTA0,Tyr3,Thr8]octreotate (DOTATATE) therapy showed a very promising therapy response rate, with mild bone marrow suppression and low nephrotoxicity [14, 15]. The impact of this type of treatment on overall survival (OS) of patients with NET has been shown in a prospective, randomized, phase III trial evaluating the effect of 177Lu-DOTATATE in combination with octreotide LAR 30 mg vs octreotide LAR 60 mg in midgut tumors [16].
At the same time, attempts were made to combine the complementary characteristic of the beta emitters with a long and short range of action to improve treatment of tumors of various sizes. In a rat model studied, by De Jong et al., a threefold increase in survival was demonstrated when using simultaneous a mix of 50% 177Lu-DOTATATE and 50% 90Y-DOTATOC [17, 18].
Based on these results, we decided to check the effects of simultaneous 90Y/177Lu-DOTATATE (tandem therapy) in humans. The comparison of simultaneous 90Y/177Lu-DOTATATE and 90Y-DOTATATE alone was investigated, showing prolongation of OS in the group of patients treated with tandem therapy, with a comparable safety level of both methods [19]. The data of single center trial using simultaneous 90Y/177Lu-DOTATATE was published in 2017 [20]. These results enabled inclusion of PRRT using 90Y/177Lu-DOTATATE tandem therapy to the treatment protocols used in Poland and the recommendations of the Polish Network of Neuroendocrine Tumors [1, 2, 21].
In the literature, it is limited data on use simultaneous 90Y/177Lu-DOTATATE. To confirm our previous results, we examine efficacy of simultaneous 90Y/177Lu-DOTATATE in lager group of patients in multicenter trial.
The aim of the present work was in a multicenter trial to determine the therapeutic efficacy and toxicity of the simultaneous combination treatment, tandem 90Y /177Lu-DOTATATE, in patients with disseminated NET. The main endpoints were progression-free survival (PFS), OS, and treatment safety. Additionally, we assessed survival parameters relative to disease grading and primary tumor location.
Material and methods
This was a multi-institution study approved by the ethical committees of Medical University of Warsaw, Military Institute of Medicine, Warsaw, and University of Medical Sciences, Poznan. We retrospectively analyzed a consecutive cohort of patients who had well-differentiated NET with Ki-67 index ≤20% and underwent tandem 90Y /177Lu-DOTATATE therapy. All patients gave written informed consent.
Patients
103 patients (39 males and 64 females at the mean age (± SD) of 56.7 ± 11.5 years) with diffused, histologically confirmed NET were included in the study.
Tumors were categorized according to the current TNM staging and grading system for NET.
All patients showed the following inclusion criteria:
Histological confirmation of NET G1 or G2 tumor; metastatic, inoperable disease.
Preserved hematological, liver and renal parameters: hemoglobin ≥10 g/dL, white blood cell (WBC) count ≥3 × 109/L, platelet count ≥90 × 109/L, bilirubin ≤1.5 × upper limit of normal (ULN), ALT <2.5 × ULN, and estimated creatinine clearance (CrCl) > 40 mL/min.
Positive somatostatin receptor imaging (SRI): PET/CT using 68Ga-DOTATATE or scintigraphy using 99mTc-HYNICTOC (Tektrotyd, POLATOM, Poland), with Krenning score ≥ 2/3.
Karnofsky index ≥70, ECOG performance status ≤2.
Age > 18 years.
Life expectancy >3 months.
No pregnancy or lactation.
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Study treatment and radiopeptide administration
All patients received simultaneous intravenous infusions of a mix of 90Y/177Lu-DOTATATE, comprising 50% radioactivity of 90Y-DOTATATE and 50% radioactivity of 177Lu-DOTATATE (administered activity per one cycle: 3.7 GBq: 1.85 GBq 90Y-DOTATATE +1.85 GBq 177Lu-DOTATATE or 2.96 GBq: 1.48GBq 90Y-DOTATATE +1.48-GBq 177Lu-DOTATATE).
As 2.5% arginine and 2.5% lysine solution was not available, a 1000 mL of mixed amino acid infusion (1000 mL, Vamin 18 Fresenius Kabi, Aminomel 12,5 Baxter, Nephrotec 10%, Fresenius Kabi) and Ringer’s solutions (500 mL) were used for renal protection, 200 mL over 30–60 min immediately before the therapy, continued during the therapy and for 8 h after the therapy [22].
Before administration of the radiopharmaceutical, ondansetron (8 mg, Zofran, Glaxo Wellcome, Atossa, Anpharm SA.) was given intravenously to prevent nausea and vomiting.
The tandem therapy 90Y/177Lu-DOTATATE was prepared as previously described using 90Y and 177Lu (ItraPol, LutaPol, POLATOM, Poland) [19, 20]. In patients receiving long-acting somatostatin analog therapy, PRRT was performed 4–5 weeks after injection of octreotide (Sandostatin LAR; Novartis) and 5–7 weeks after injection of lanreotide (Somatuline Autogel; Ipsen). Somatostatin analogue therapy was continued between PRRT cycles. The interval between completing of chemotherapy and PRRT was longer than 3 months.
Post-therapy imaging
Post-therapy imaging was performed 24 h after the therapy to assess biodistribution. The acquisition was made with an energy window ±10% centered on 177Lu photopeaks (208 keV), as described previously [19, 20].
Assessment of treatment results and clinical benefits
The primary end points were progression-free survival, which was defined as the time from 90Y/177Lu-DOTATATE treatment to documented disease progression or death from any cause and safety profile. Secondary end points included the objective response rate and overall survival.
All patients underwent staging by contrast-enhanced CT or MRI and SRI using 99mTc-HYNICTOC or 68Ga-DOTATATE PET/CT.
Long term response monitoring following PRRT completion was undertaken at 3–6, 12 months and every 12 months thereafter, using blood markers CT or MRI and SRI following the same protocols as had been used prior to therapy.
Blood tests for complete blood cell count and kidney and liver function parameters were repeated every 7–21 days after each therapy cycle, and 3, 6, 12 and every 12 months after completing the therapy. Toxicity was recorded using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Kidney function was estimated using the Modification of Diet in Renal Disease formula.
Statistical methods
Mean values and standard deviations, medians and quartiles or frequencies depending on the parameter distribution were used to summarize patients characteristics.
The progression free survival (PFS) was defined as the time from the start of radioisotope treatment to the first evidence of progression by imaging criteria (SRI and/or CT/MRI).
Overall survival from diagnosis was defined as the time from the first diagnosis of the tumor to death from any cause (OS-D). OS from the start of treatment was defined as the time from the first cycle of 90Y/177Lu-DOTATATE treatment to death from any cause (OS-T).
OS, PFS, probability of 2-year and 5-year OS, and 2-year risk of progression were calculated using the Kaplan-Meier estimator and compared using the log-rank test [23]. Calculations were performed using GraphPad PRISM 5 (GraphPad Software Inc).
Results
Patient characteristics
103 patients underwent PRRT between February 2006 and July 2017.
Of 103 patients, 32 patients were diagnosed with a pancreatic neuroendocrine primary tumor, 29 with a tumor originating from small bowel, 20 with a tumor originating from large bowel, 4 with a bronchopulmonary tumor, 12 had tumors of unknown primary origin and 6 had tumors arising from other sites (1 epiglottis, 1 multiple endocrine neoplasia [MEN] type 1, 1 von Hippel-Lindau syndrome [VHL] with pancreatic tumor, 2 multiple paragangliomas, 1 retroperitoneal area). 39 patients had low grade tumor NET G1 and 64 had intermediate grade tumor G2 NET.
Prior therapies before commencing PRRT were as follows: 77 patients underwent surgery with primary tumor resection and additionally one patient underwent liver transplantation due to metastases, 75 patients received long-acting somatostatin analog therapy and 23 patients received chemotherapy. Detailed patient data are shown in Table 1. Three patients were lost to follow up and are excluded from further analysis.
90Y/177Lu-DOTATATE tandem therapy
Patients were usually treated with 4 cycles of 3.7 GBq of 90Y/177Lu-DOTATATE per injection at 6–12 weeks intervals, depending on clinical status, laboratory results and radiopharmaceutical availability. In patients with hematological or kidney deterioration during treatment or in patients with low body mass (below 50 kg) the injected activity was reduced to 2.96 GBq 90Y/177Lu-DOTATATE or they received only 3 cycles.
Three patients received 2 cycles, one because of progression during therapy, two because of patient choice. Eight patients received 3 doses with cumulative activity of 11.1 GBq. The remaining patients (89%) completed their planned four cycles of 90Y/177Lu-DOTATATE treatment with administered cumulative activity of 12.95–14.8 GBq.
All patients with NET-related syndrome received long-acting somatostatin analogs, before and during PRRT with 4–5 weeks after injection of octreotide and 5–7 weeks after injection of lanreotide.
Imaging response
Imaging response was based on the radiological evaluation according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria and SRI.
It was performed at the first available checkpoint 3–6 months after therapy with partial response (PR) in 17(17%) patients, stable disease (SD) in 78 (78%) patients and progressive disease (PD) in 5 (5%) patients. At 3–6 months follow up complete response (CR) was not observed, no patients died.
Additionally, the imaging evaluation was performed one year after therapy. At one-year follow up eight patients died, CR was seen in 2 (2%) patients, PR in 20 (22%) patients, SD in 59 (64%) patients and PD in 11 (12%)patients.
At 3–6 months vs 12 months follow-up, the disease control rate was 95% vs 88%. The objective response rate (ORR), defined as in the proportion of patients achieving PR or CR, was 17% vs 24%, receptively.
Survival parameters
The median follow-up duration was 44.1 months (interquartile range IQ: 30.5 to 74.6 months). 41 patients died during follow-up.
Overall survival was calculated for all patients and a Kaplan-Meier survival curve was generated for all evaluable patients in this study. PFS was also calculated (including deaths).
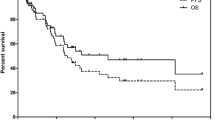
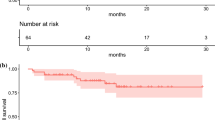
The median overall survival from diagnosis (OS-D) was 127.4 months, and overall survival from the time of PRRT (OS-T) was 89.5 months. PFS was 29.9 months (Fig.1).
The observed 2-year risk of progression was 42%. The probability of 2-year and 5-year overall survival from start of therapy was 89% and 62%, respectively.
Correlation of grading and survival parameters
When the proliferation index of tumors (G1, n = 37; G2, n = 63) was taken into consideration, Kaplan–Meier curve analysis showed a significant impact on PFS and OS-T.
Patients with G1 tumors had a significantly longer PFS, median PFS 59.3 months, compared with patients with G2 tumors, median PFS 24.3 months (P = 0.016) (Fig.2).
Median OS-T for patients with G2 tumors was 79.9 months, while the median OS-T for patients with G1 tumors was not reached (P = 0.026) (Fig.3).
The median overall survival time from diagnosis (OS-D) was 102.6 months for G2 tumors, and not defined for G1 tumors. This difference was not statistically significant on the Kaplan–Meier curve.
The observed overall 2-year survival from start of PRRT in G1 vs G2 patients was 91% vs 88%, and 5-year survival was 69% vs 56%, respectively. The 2-year risk of progression was 33% in G1 patients and 51% in G2 patients. Examples of therapeutic effect are presented in Figs. 4 and 5.
Example of tandem 90Y/177Lu-DOTATATE therapy effect. A 62-year-old woman with non-functional pancreatic NET G2 and multiple liver metastases, after surgical treatment. 68Ga- DOTATATE PET/CT before treatment showing uptake in the metastatic lesions in the liver. At 12 and 36 months of follow up, 68Ga- DOTATATE PET/CT showed complete treatment response within liver metastases. Additionally, uptake in the uncinate process of the pancreas is visible, with no abnormality by endoscopic ultrasound (EUS).
Example of tandem 90Y/177Lu-DOTATATE therapy effect. A 63y-old woman with non-resectable, non-functional pancreatic NET G1 with metastases to lymph nodes, bones and the liver. 99mTc-HYNIC-TOC before treatment with high uptake in the primary pancreas tumor and in the metastatic lesions as well. Six- month follow up with 99mTc-HYNIC-TOC showed partial remission in the primary tumor and retroperitoneal lymph nodes.
Effect of primary tumor site
The effect of the primary disease site was also analyzed. Tumors were -grouped into those arising from the pancreas, small bowel, and large bowel primary sites.
Pancreatic NET
For pancreatic NET (n = 31) PFS was 30.8 OS-T was 94 months and OS-D was 130.4 months.
The 2-year survival from start of PRRT for pancreatic NET was 84% and 5-year overall survival was 43%.
The 2-year risk of progression for pancreatic NET was 45%.
Small bowel NET
For small bowel NET (n = 28) PFS was 30.3 months, and OS-T was 61.9 months. OS-D was 89.2 months.
The 2-year survival from start of PRRT for small bowel NET was 89%, and 5-year overall survival was 29%.
The 2-year risk of progression for small bowel NET 46%.
Large bowel NET
For large bowel NET (n = 20), PFS was 40.6 months, and OS-T was 131.2 months. OS-D was not reached.
The 2-year survival from start of PRRT for large bowel NET was 100%, and 5-year overall survival was 50%.
The 2-year risk of progression for large bowel NET was 45%.
The survival parameters depend on primary tumor location
A statistically significant difference in OS-T was observed between primary tumors located in pancreas vs large bowel (p = 0.033) and small bowel vs large bowel (p = 0.009); in OS-D small bowel vs large bowel NET (p = 0.047). The difference between other locations and survival parameters was not statistically significant.
The effect of the primary disease site and grading revealed statically significant difference in OS-T for pancreatic NET G1 and G2. Effects of primary tumor site and grading are presented in Tables 2, 3 and 4.
Toxicity of 90Y/177Lu-DOTATATE tandem therapy
Treatment was well tolerated by patients and no severe adverse events occurred.
The most common transient acute side effects were nausea (in approximately half of the patients) and asthenia. No patient had a carcinoid crisis following the treatment or any acute reaction during radionuclide therapy infusion.
During the treatment, a transient decrease in platelet and WBC count was observed in three patients (3%).
After completing PRRT, toxicity grade 1 leukocytopenia was found in 17 patients (18%) and grade 2 in two patients (2%); grade 1 thrombocytopenia in 5 patients (5%) and grade 2 in two patients (2%); grade 1 decrease in hemoglobin level was seen in 12 patients (13%) and grade 2 in five patients (5%). One patient (1%) developed myelodysplastic syndrome (MDS). Possible predisposing risk factors for MDS included: previous 2 lines of chemotherapy and high tumor load with hepatic, lymph node and bone metastases. No other grade 3 and 4 hematotoxicity was observed.
Patients who experienced a degree of nephrotoxicity had at least 2 risk factors (diabetes, hypertension, age, previous chemotherapy, chronic NSAID treatment). Only 3 of them had normal kidney function before therapy. Grade 1 nephrotoxicity was seen in 14 patients (15%), and grade 2 nephrotoxicity was seen in 3 patients (3%). No Grade 3 or 4 nephrotoxicity was observed.
To date, no hepatic toxicity of any grade or other clinically significant toxicity has been observed.
Discussion
PRRT using radiolabeled somatostatin analogs initially with 111In, afterwards 90Y and now 177Lu has been used for more than 20 years. Numerous phase I and phase II studies have shown favorable PFS and OS in NET patients compared to historical controls [9,10,11,12,13,14].
The NETTER-1, first phase III multicenter, stratified, open, randomized, controlled, parallel-group study demonstrated that [177Lu-DOTA,Tyr3]octreotate significantly improved PFS compared to Octreotide LAR (Sandostatin® LAR; Novartis; 60 mg) in patients with advanced midgut NET. The estimated PFS in the 177Lu-DOTATATE plus Octreotide LAR group was 40 months vs 8.4 months in the Octreotide LAR arm [16] which is comparable with reported data from non-randomized trials.
These results clearly showed that patients with midgut NET treated by PRRT with 177Lu-DOTATATE had a survival advantage compared to other forms of treatment including targeted therapy with mTOR or kinase inhibitor [24, 25].
Research continues into the use of 90Y and 177Lu, both as single agents and in combination for PRRT. 90Y and 177Lu have different physical properties, such as the half-life and energy of the β-particles.
Based on Marion de Jong’s study, we used a 50:50 combination of 90Y and 177Lu activity for tandem PRRT. This protocol was described in 2006, and continued later [17,18,19,20].
Recently, the size of individual lesions and optimal radionuclide choice was investigated in dosimetry studies and models. The radius within which 90% of energy is deposited in 5.82 mm for 90Y and in 0.62 mm for 177Lu, clearly suggest that 90Y is not an adequate isotope for eradicating small or micrometastases [26]. 177Lu delivers significantly higher normalized absorbed doses to 1-cm, 1-mm and 100-μm lesions than 90Y (152 Gy vs 96 Gy, 104 Gy vs 13.3 Gy and 24.5 Gy vs 1.36 Gy, respectively) [26]. However, the dose delivered by 90Y increased rapidly when sphere size increased [26].
The optimal composition of isotopes was investigated. The composition for tandem therapy based on 50% 90Y and 50% 177Lu is probably not the best choice. In one Polish center, proportion 1.85 GBq of 90Y and 5.55 GBq of 177Lu was examined, but it resulted in significant nephro- and hematotoxicity (not published data).
Based on dosimetry studies, a combination of 2 GBq 90Y-DOTATOC and 4 GBq 177Lu-DOTATOC seems to be better for patients with larger and moderate size lesions [27].
Consequently, in patients with tumors of various sizes and heterogeneous receptor distribution, the use of radionuclide combinations may lead to higher efficacy compared to the use of a single radioisotope [18, 27, 28].
There are currently no randomized trial data comparing the outcomes of the 90Y and 177Lu PRRT combination to outcomes after treatment with either of those isotopes alone.
Consequently, though potential efficacy has been reported, the benefit of this combination of radionuclides in humans with NET requires further study [19, 20, 29,30,31,32],
Our early results of tandem therapy are encouraging, showing longer overall survival after the combination 90Y/177Lu -DOTATATE treatment compared to the group treated with 90Y-DOTATATE alone. The overall survival for 90Y-DOTATATE was 26.3 months and for 90Y/177Lu -DOTATATE was not reached [19]. Long-term follow-up after tandem 90Y/177Lu -DOTATATE therapy showed PFS of 32.2 months and OS of 82 months [20]. The simultaneous use of 90Y/177Lu –DOTATATE in a multicenter study showed PFS 29.9 months and OS 89.5 months.
The use of the combination of both radionuclides has been corroborated by other researchers. Seregni et al. tested a much higher injected activity and different proportion of 177Lu DOTATATE (5.55 GBq) and 90Y DOTATATE (2.6 GBq) which induced better objective responses in 42.3% of patients, but shorter median PFS 24 months by comparison with our study [33]. Villard et al. and Dumont et al. investigated a different somatostatin analog, DOTATOC. Both groups showed similar and comparable results to our own findings with longer OS after treatment with 90Y/177Lu-DOTATOC vs 90Y-DOTATOC (in the cohort study, OS was 66.1 vs 47.5 months, and in patients with gastrinoma, OS was 60.2 vs 27.0 months, respectively) [29, 30]. However, both authors use combination therapy utilizing alternating cycles of 90Y-DOTATOC and 177Lu-DOTATOC, not using both isotopes simultaneously.
2 papers have been published in the literature regarding studies with three treatment arms, comparing 90Y and 77Lu alone to the combination of both radionuclides [31, 32]. Neither of these was a randomized study.
A German multicenter study showed reduced PFS and OS in a group of patients treated with 90Y or 177Lu only compared to the combination of 90Y with 177Lu as combined or sequential therapy [31].
Superior response to combination therapy was confirmed in a multivariate survival analysis, in which 90Y-DOTATOC plus 177Lu-DOTATOC treatment (combined or sequential) was correlated with a longer survival compared to 90Y-DOTATOC (66.1 vs 47.5 months; n = 1358; p < 0.001) or 177Lu-DOTATOC alone (66.1 vs 45.5 months; n = 390; p < 0.001) [34].
In a recently published paper in 1048 patients treated with 90Y and 177Lu either as a combination of both radionuclides in one cycle (TANDEM) or sequentially (DUO), or with 90Y and 77Lu alone, best OS (66 months) was achieved with the combination of 90Y and 177Lu for PRRT. Treatment with 177Lu alone resulted in the next longest survival time (44 months) while the shortest time of survival (24 months) was observed in patients treated only with 90Y. The PFS achieved for these 3 patient groups was 24, 17 and 7 months, respectively [32]. Despite smaller group sizes, we obtained similar or even better survival results.
The survival gain we have observed in our study may reflect the relatively high number of NET G2 subjects in our patient population. . In our study,the proportion of G1/G2 was 37%/63% in comparison to the NETTER-1 study where the proportion G1/G2 was reverse 66%/34% [16]. In patients with G2 tumors, 18F-FDG PET/CT imaging is a good prognostic indicator, but this is not reimbursed for NET patients in Poland [35, 36].
In our group of patients, we observed better PFS and OS-T for NET G1 patients. This is contrary to observations published by Horsch et al. [31] but consistent with Baum et al. who reported longer overall survival in NET G1 patients and concluded that tumor grade was a strong predictor of overall survival [32].
Published articles had limited data concerning the results of the PRRT in correlation with the origin of primary tumor. Horsch D et al. observed that patients with small bowel NET were significantly less likely to die than patients with other primaries, whereas there was no significant difference in the PFS [31]. The same result was obtained by Baum et al. where the small intestine location had longer OS than NETs arising from other primary sites. [32]. In our previous single center analysis and now from our multicenter study, we observed the longest PFS and OS-T in NET arising from the large bowel [20]. Patients with midgut tumors according to results of PROMID and CLARINET studies, are first treated for a long time with long acting somatostatin analogues. Patients with a other NET localization are more likely to induce to more aggressive treatment including the PRRT. This can have significant effects on survival parameters.
From the clinical point of view, treatment related toxicity is an essential consideration. Published studies have reported several serious side effects of PRRT.
Before the introduction of amino-acid infusion, nephrotoxicity was common and was dose limiting, particularly in association with 90Y labeled peptide therapy...
In the NETTER-1 trial with 177Lu-DOTATATE, no evidence of renal toxic effects were reported during 14 months follow-up. Nephrotoxicity is a well-known side-effect, requires a minimum follow-up of 6 months to be observed, more likely 1 year [12, 13, 37,38,39]. In our group treated with tandem 90Y/177Lu-DOTATATE, grade 3 and 4 toxicity was not observed, even with longer follow-up (median 44.1 months) [16].
Hematological toxicity is usually transient. In the NETTER-1 trial, grade 3 or 4 neutropenia, thrombocytopenia, and lymphopenia were reported in 1%, 2%, and 9% of patients, respectively [16]. In our group, long-term hematotoxicity in the form of MDS was noted in one patient (1%), which is comparable to the results reported in the literature [15, 37]. No other grade 3 and 4 hematotoxicity was observed. No hepatotoxicity or others serious adverse events were noted.
The present study has some strengths and limitations. The main limitation of the present study is its retrospective design, relatively small patient cohort and lack of dosimetry.
Seregni et al. [28] reported the results of a dosimetry phase II study in a group of patients refractory to conventional therapy. By comparison with our own study, higher administered activities of 177Lu DOTATATE (5.55 GBq) and 90Y DOTATATE (2.6 GBq) did not result in kidney damage and the cumulative BED values were below the toxicity limit in the majority of patients [33]. The dosimetry implications related to the size of the lesion and possible kidney or bone marrow toxicity are certainly important but are not the only variables to consider. Recent published studies have shown that pre existing risk factors for kidney damage increase the probability of PRRT toxicity, so this is a group of patients who might benefit from pre treatment dosimetry studies. On the other hand, dosimetry studies have demonstrated that not only total administered activity per cycle but also the total number of cycles play an important role in PRRT toxicity, lower toxicity being associated with lower cumulative activities [37]. In our treatment protocols, we applied half of the former use injected activity of 90Y and much lower activity of 177Lu in smaller cohort. Nevertheless, we obtained similar survival rates with a low incidence of side effects.
Limited dosimetry data that we obtained at the beginning of therapy showed absorbed doses for kidney and bone marrow comparable to other publications (for kidney 2.6Gy/GBq for 90Y and 0.7 Gy/GBq for 177Lu; for bone marrow below 0.2 Gy per cycle) [33, 38]. On the basis of theses reassuring data and busy day clinical practice, this investigation was not continued.
The low incidence of serious adverse events, high probability of disease control and long PFS and OS constitute real benefits for NET patients. However, a limited population size, lack of control group (treated with yttrium or lutetium alone) and lack of randomization will inevitably affect the strength of our conclusions.
The NETTER-1 trial strongly confirmed the effectiveness of PRRT, but the optimal radiolabel and role of combination therapy remains controversial. There is no randomized three-arm trial evidence comparing outcomes for simultaneous 90Y/177Lu-DOTATATE or sequential 90Y- and 177Lu-DOTATATE treatment compared to single isotope [31, 32]. This suggests that it need future evaluation in lager group of patients.
Conclusions
These results of a multicenter trial indicate that tandem radioisotope 90Y/177Lu-DOTATATE therapy for patients with metastatic neuroendocrine tumors (G1 and G2) is highly effective and safe, with limited side effects. We have also demonstrated statistically significant favorable outcomes of tandem 90Y/177Lu-DOTATATE therapy in patients with NET G1 compared to NET G2.
References
Kos-Kudła B, Blicharz-Dorniak J, Strzelczyk J, et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the polish network of neuroendocrine Tumours). Endokrynol Pol. 2017;68:79–110. https://doi.org/10.5603/EP.2017.0015.
Bednarczuk T, et al. Neuroendocrine neoplasms of the small intestine and appendix - management guidelines (recommended by the polish network of neuroendocrine Tumours). Endokrynol Pol. 2017;68:223–36. https://doi.org/10.5603/EP.2017.0018.
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Öberg K, Knigge U, Kwekkeboom D, et al. On behalf of the ESMO guidelines working group. Neuroendocrine gastro-enteropancreatic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:124–30.
Trofimiuk-Muldner M, Lewkowicz E, Wysocka K, et al. Epidemiology of gastroenteropancreatic neuroendocrine neoplasms in Krakow and Krakow district in 2007-2011. Endokrynol Pol. 2017;68:42–6.
Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic Enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33.
Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report fromthe PROMID study group. J Clin Oncol. 2009;27:4656–63.
Delle Fave GF, O'Toole D, Sundin A, et al. ENETS consensus guidelines update for Gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016;103:119–24.
Valkema R, de Jong M, Bakker WH, et al. Phase I study of peptide receptor radionuclide therapy with [111In-DTPA0]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–22.
Anthony LB, Woltering EA, Espanan GD, Cronin MD, Maloney TJ, McCarthy KE. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32:123–32.
Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4GBq 90Y-DOTATOC. J Nucl Med. 2002;43:610–6.
Bodei L, Cremonesi M, Grana C, et al. Receptor radionuclide therapy with 90Y[DOTA]0-Tyr3-octerotide(90Y- DOTATOC) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2004;31:1038–46.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46:62S–6S.
Kwekkeboom D, Bakker W, Kam BLR, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med. 2003;30:417–22.
Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Strosberg J, El-Haddad G. Wolin E et al phase 3 trial of 177Lu-Dotatate for Midgut neuroendocrine tumors. N Engl J Med. 2017;12:125–35. https://doi.org/10.1056/NEJMoa1607427.
De Jong M, Bernard HF, Breeman WA, et al. Combination of 90Y- and 177Lu- labeled somatostatin analogs is superior for radionuclide therapy compared to 90Y- or 177Lu-labeled analogs only. J Nucl Med. 2002;43:123P–4P.
de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med. 2005;46:13S–7S.
Kunikowska J, Królicki L, Hubalewska-Dydejczyk A, Mikołajczak R, Sowa-Staszczak A, Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging. 2011;38:1788–97.
Kunikowska J, Pawlak D, Bąk MI, Kos-Kudła B, Mikołajczak R, Królicki L. Long-term results and tolerability of tandem peptide receptor radionuclide therapy with 90Y/177Lu-DOTATATE in neuroendocrine tumors with respect to the primary location: a 10-year study. Ann Nucl Med. 2017;31:347–56. https://doi.org/10.1007/s12149-017-1163-6.
Kos-Kudła B, Blicharz-Dorniak J, Handkiewicz-Junak D, et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the polish network of neuroendocrine Tumours). Endokrynol Pol. 2013;64:418–43.
Rolleman EJ, Valkema R, de Jong M, et al. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13 Errata w: N Engl J Med 2011; 364:1082.
Yao JC, Shah MH, Ito T, et al. RAD001 in advanced neuroendocrine tumors, third trial (RADIANT-3) study group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23.
Hindié E, Zanotti-Fregonara P, MQuinto MA, Morgat C, Champion C. Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various sizes: implications for radiopharmaceutical therapy. J Nucl Med. 2016;57:759–64. https://doi.org/10.2967/jnumed.115.170423.
Cieciera M, Kratochwil C, Moltz J, et al. Semi-automatic 3D-volumetry of liver metastases from neuroendocrine tumors to improve combination therapy with 177Lu-DOTATOC and 90Y-DOTATOC. Diagn Interv Radiol. 2016;22:201–6.
Siegel JA, Stabin MG. Absorbed fractions for electrons and beta particles in spheres of various sizes. J Nucl Med. 1994;35:152–6.
Villard L, Romer A, Marincek N, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012;30:1100–6.
Dumont RA, Seiler D, Marincek N, et al. Survival after somatostatin based radiopeptide therapy with (90)Y-DOTATOC vs. (90)Y-DOTATOC plus (177)Lu-DOTATOC in metastasized gastrinoma. Am J Nucl Med Mol Imaging. 2014;5:46–55.
Horsch D, Ezziddin S, Haug A, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur J Cancer. 2016;58:41–51.
Baum R, Kulkarni H, Singh A, et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932–50.
Seregni E, Maccauro M, Chiesa C, et al. Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy. Eur J Nucl Med Mol Imaging. 2014;41:223–30.
Radojewski P, Dumont R, Marincek N, et al. Towards tailored radiopeptide therapy. Eur J Nucl Med Mol Imaging. 2015;42:1231–7.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85.
Severi S, Nanni O, Bodei L. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:881–8.
Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. https://doi.org/10.1007/s00259-014-2893-5 Epub 2014 Oct 2.
Cremonesi M, Ferrari ME, Bodei L, et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur J Nucl Med Mol Imaging. 2018;45:2426–41. https://doi.org/10.1007/s00259-018-4044-x.
Kunikowska J, Królicki L, Sowa-Staszczak A, Pawlak D, Hubalewska-Dydejczyk A, Mikołajczak R. Nephrotoxicity after PRRT - still a serious clinical problem? Renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATATE and 90Y/177Lu-DOTATATE. Endokrynol Pol. 2013;64:13–20.
Acknowledgments
This study was partially supported by Research Grants (6 P05 2004 C/6453 and 4/85195/1210/529) from the Ministry of Health and Ministry of Education.
We thank the radiochemistry group at the Nuclear Medicine, the nursing staff and the technologists from the Medical University of Warsaw, Military Institute of Medicine and Poznan University of Medical Sciences for their support.
Special thanks for Prof. Valerie Lewington from Guy’s and St Thomas’ NHS Foundation Trust, London for comments and English correction and Radosław Kuliński from Medical University of Warsaw for dosimetry data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest in relation to this article.
This article does not contain any studies with animals performed by any of the authors.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Digestive tract
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kunikowska, J., Zemczak, A., Kołodziej, M. et al. Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects - polish multicenter experience. Eur J Nucl Med Mol Imaging 47, 922–933 (2020). https://doi.org/10.1007/s00259-020-04690-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04690-5