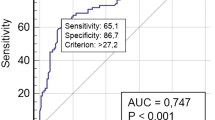
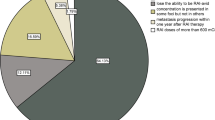
Abstract
Purpose
We investigated whether predictive clinicopathologic factors can be affected by different response criteria and how the clinical usefulness of radioactive iodine (RAI) therapy should be evaluated considering variable factors in patients with differentiated thyroid carcinoma (DTC).
Methods
A total of 1563 patients with DTC who underwent first RAI therapy after total or near total thyroidectomy were retrospectively enrolled from 25 hospitals. Response to therapy was evaluated with two different protocols based on combination of biochemical and imaging studies: (1) serum thyroglobulin (Tg) and neck ultrasonography (US) and (2) serum Tg, neck US, and radioiodine scan. The responses to therapy were classified into excellent and non-excellent or acceptable and non-acceptable to minimize the effect of non-specific imaging findings. We investigated which factors were associated with response to therapy depending on the follow-up protocols as well as response classifications. Multivariate logistic regression analysis was performed to identify factors significantly predicting response to therapy.
Results
The proportion of patients in the excellent response group significantly decreased from 76.5 to 59.6% when radioiodine scan was added to the follow-up protocol (P < 0.001). Preparation method (recombinant human TSH vs. thyroid hormone withdrawal) was a significant factor for excellent response prediction evaluated with radioiodine scan (OR 2.129; 95% CI 1.687–2.685; P < 0.001) but was not for other types of response classifications. Administered RAI activity, which was classified as low (1.11 GBq) or high (3.7 GBq or higher), significantly predicted both excellent and acceptable responses regardless of the follow-up protocol.
Conclusions
The clinical impact of factors related to response prediction differed depending on the follow-up protocol or classification of response criteria. A high administered activity of RAI was a significant factor predicting a favorable response to therapy regardless of the follow-up protocol or classification of response criteria.


Similar content being viewed by others
References
Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, Gafni A, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin N Am. 2008;37:457–80 x.
Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–45.
Lin HW, Bhattacharyya N. Survival impact of treatment options for papillary microcarcinoma of the thyroid. Laryngoscope. 2009;119:1983–7.
Al-Qahtani KH, Al Asiri M, Tunio MA, Aljohani NJ, Bayoumi Y, Fatani H, et al. Adjuvant Radioactive iodine 131 ablation in papillary microcarcinoma of thyroid: Saudi Arabian experience [corrected]. J Otolaryngol Head Neck Surg. 2015;44:51.
Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015;100:1748–61.
Pacini F. Prospective study confirms that radioiodine remnant ablation is not necessary in low-risk differentiated thyroid cancer. Eur Thyroid J. 2016;5:7–8.
Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29:461–70.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133.
Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–20.
Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–63.
Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89:3668–76.
Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–62.
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–85.
Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–73.
Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, Bardet S, et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. 2018;6:618–26.
Verburg FA, Mader U, Reiners C, Hanscheid H. Long-term survival in differentiated thyroid cancer is worse after low-activity initial post-surgical 131I therapy in both high- and low-risk patients. J Clin Endocrinol Metab. 2014;99:4487–96.
Verburg FA, Aktolun C, Chiti A, Frangos S, Giovanella L, Hoffmann M, et al. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43:1001–5.
Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29:461–70.
Sabra MM, Grewal RK, Ghossein RA, Tuttle RM. Higher administered activities of radioactive iodine are associated with less structural persistent response in older, but not younger, papillary thyroid cancer patients with lateral neck lymph node metastases. Thyroid. 2014;24:1088–95.
Maxon HR 3rd, Englaro EE, Thomas SR, Hertzberg VS, Hinnefeld JD, Chen LS, et al. Radioiodine-131 therapy for well-differentiated thyroid cancer--a quantitative radiation dosimetric approach: outcome and validation in 85 patients. J Nucl Med. 1992;33:1132–6.
Flux GD, Haq M, Chittenden SJ, Buckley S, Hindorf C, Newbold K, et al. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:270–5.
Jentzen W, Hoppenbrouwers J, van Leeuwen P, van der Velden D, van de Kolk R, Poeppel TD, et al. Assessment of lesion response in the initial radioiodine treatment of differentiated thyroid cancer using 124I PET imaging. J Nucl Med. 2014;55:1759–65.
Grewal RK, Larson SM, Pentlow CE, Pentlow KS, Gonen M, Qualey R, et al. Salivary gland side effects commonly develop several weeks after initial radioactive iodine ablation. J Nucl Med. 2009;50:1605–10.
van der Horst-Schrivers AN, Sluiter WJ, Muller Kobold AC, Wolffenbuttel BH, Plukker JT, Bisschop PH, et al. Recombinant TSH stimulated remnant ablation therapy in thyroid cancer: the success rate depends on the definition of ablation success--an observational study. PLoS One. 2015;10:e0120184.
Jeong SY, Lee SW, Kim WW, Jung JH, Lee WK, Ahn BC, et al. Clinical outcomes of patients with T4 or N1b well-differentiated thyroid cancer after different strategies of adjuvant radioiodine therapy. Sci Rep. 2019;9:5570.
Lim DJ, O JH, Kim MH, Kim JH, Kwon HS, Kim SH, et al. Clinical significance of observation without repeated radioiodine therapy in differentiated thyroid carcinoma patients with positive surveillance whole-body scans and negative thyroglobulin. Korean J Intern Med. 2010;25:408–14.
Zhang D, Tang J, Kong D, Cui Q, Wang K, Gong Y, et al. Impact of gender and age on the prognosis of differentiated thyroid carcinoma: a retrospective analysis based on SEER. Horm Cancer. 2018;9:361–70.
Acknowledgments
KSNM Thyroid Cancer CTN Retrospective Cohort Study Group: Seong Young Kwon and Subin Jeon (Chonnam National University Medical School and Hwasun Hospital, Jeonnam); Sang-Woo Lee and Shin Young Jeong (Kyungpook National University, School of Medicine and Chilgok Hospital, Daegu); Eun Jung Kong (Yeungnam University Medical School and Hospital, Daegu); Keunyoung Kim (Pusan National University, Busan); Byung Il Kim (Korea Institute of Radiological & Medical Sciences, Seoul); Jahae Kim (Chonnam National University Hospital, Gwangju); Heeyoung Kim (Kosin University Gospel Hospital, Busan); Seol Hoon Park and Minjung Seo (Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan); Jisun Park (Inje University Busan Paik Hospital, Busan); Hye Lim Park (Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul); So Won Oh (Seoul National University Boramae Medical Center, Seoul); Kyoung Sook Won (Keimyung University Dongsan Hospital, Daegu); Young Hoon Ryu (Yonsei University Gangnam Severance Hospital, Seoul); Joon-Kee Yoon (Ajou University School of Medicine, Suwon); Soo Jin Lee (Hanyang University Medical Center, Seoul); Jong Jin Lee (Asan Medical Center, University of Ulsan College of Medicine, Seoul); Ari Chong (Chosun University Hospital, Gwangju); Young Jin Jeong (Dong-A University Hospital, Busan); Ju Hye Jeong (Kyungpook National University Hospital, Daegu); Young Seok Cho (Samsung Medical Center, Seoul); Arthur Cho (Yonsei University College of Medicine, Seoul); Gi Jeong Cheon and Seunggyun Ha (Seoul National University College of Medicine, Seoul); Eun Kyoung Choi (Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul); Jae Pil Hwang (Soon Chun Hyang University Bucheon Hospital, Bucheon); and Sang Kyun Bae (Inje University Haeundae Paik Hospital, Busan)
The authors thank Ms. Jayeong Paek, Institute for Biomedical Science CNUHH, for her assistance of statistical analysis.
Funding
This study was supported by the Clinical Trial Network Program of the Korean Society of Nuclear Medicine (KSNM-CTN-2016-01-1).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The institutional review board of our institutes approved this retrospective study, and the requirement to obtain informed consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Endocrinology
Rights and permissions
About this article
Cite this article
Kwon, S.Y., Lee, SW., Kong, E.J. et al. Clinicopathologic risk factors of radioactive iodine therapy based on response assessment in patients with differentiated thyroid cancer: a multicenter retrospective cohort study. Eur J Nucl Med Mol Imaging 47, 561–571 (2020). https://doi.org/10.1007/s00259-019-04634-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04634-8