Abstract
Objective
To apply an AT (Aβ/tau) classification system to subcortical vascular cognitive impairment (SVCI) patients following recently developed biomarker-based criteria of Alzheimer’s disease (AD), and to investigate its clinical significance.
Methods
We recruited 60 SVCI patients who underwent the neuropsychological tests, brain MRI, and 18F-florbetaben and 18F-AV1451 PET at baseline. As a control group, we further recruited 27 patients with AD cognitive impairment (ADCI; eight Aβ PET-positive AD dementia and 19 amnestic mild cognitive impairment). ADCI and SVCI patients were classified as having normal or abnormal Aβ (A−/A+) and tau (T−/T+) based on PET results. Across the three SVCI groups (A−, A+T−, and A+T+SVCI), we compared longitudinal changes in cognition, hippocampal volume (HV), and cortical thickness using linear mixed models.
Results
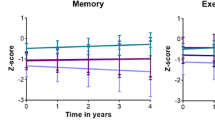
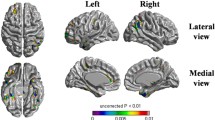
Among SVCI patients, 33 (55%), 20 (33.3%), and seven (11.7%) patients were A−, A+T−, and A+T+, respectively. The frequency of T+ was lower in A+SVCI (7/27, 25.9%) than in A+ADCI (14/20, 70.0%, p = 0.003) which suggested that cerebral small vessel disease affected cognitive impairments independently of A+. A+T−SVCI had steeper cognitive decline than A−SVCI. A+T+SVCI also showed steeper cognitive decline than A+T−SVCI. Also, A+T−SVCI had steeper decrease in HV than A−SVCI, while cortical thinning did not differ between the two groups. A+T+SVCI had greater global cortical thinning compared with A+T−SVCI, while declines in HV did not differ between the two groups.
Conclusion
This study showed that the AT system successfully characterized SVCI patients, suggesting that the AT system may be usefully applied in a research framework for clinically diagnosed SVCI.



Similar content being viewed by others
Data availability
Anonymous data are available to qualified investigators upon request to the corresponding author.
Abbreviations
- Aβ:
-
Amyloid-β
- SVCI:
-
Subcortical vascular cognitive impairment
- HV:
-
Hippocampal volume
- AD:
-
Alzheimer’s disease
- NIA-AA:
-
National Institute on Aging and Alzheimer’s Association
- PET:
-
Positron emission tomography
- CSF:
-
Cerebrospinal fluid
- CSVD:
-
Cerebral small vessel disease
- MCI:
-
Mild cognitive impairment
- ADCI:
-
Alzheimer’s disease–related cognitive impairment
- WMH:
-
White matter hyperintensities
- MRI:
-
Magnetic resonance imaging
- NC:
-
Normal control
- ADD:
-
Alzheimer’s disease dementia
- SUVR:
-
Standardized uptake value ratios
- ROI:
-
Region of interest
- PVE:
-
Partial volume effect
- FLAIR:
-
Fluid-attenuated inversion recovery
- GRE:
-
Gradient echo
- CMBs:
-
Cerebral microbleeds
- BAPL:
-
Brain Aβ plaque load
- SNSB:
-
Seoul Neuropsychological Screening Battery
- SVLT:
-
Seoul Verbal Learning Test
- RCFT:
-
Rey–Osterrieth Complex Figure Test
- KBNT:
-
Korean version of the Boston Naming Test
- MMSE:
-
Mini-Mental State Examination
- CDR-SB:
-
Clinical Dementia Rating sum of boxes
- ICV:
-
Intracranial volume
- ANOVA:
-
Analysis of variance
- ANCOVA:
-
Analysis of covariance
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011;7:263–269.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011;7:280–92.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47.
Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, et al. Pathogenesis of cerebral microbleeds: in vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol. 2013;73:584–93.
Kalaria RN, Erkinjuntti T. Small vessel disease and subcortical vascular dementia. J Clin Neurol. 2006;2:1–11.
Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–4.
Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011;7:436–44.
Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–6.
Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58:610–6.
Lee JH, Kim SH, Kim GH, Seo SW, Park HK, Oh SJ, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77:18–25.
Lee MJ, Seo SW, Na DL, Kim C, Park JH, Kim GH, et al. Synergistic effects of ischemia and beta-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiatry. 2014;71:412–22.
Ye BS, Seo SW, Kim GH, Noh Y, Cho H, Yoon CW, et al. Amyloid burden, cerebrovascular disease, brain atrophy, and cognition in cognitively impaired patients. Alzheimers Dement. 2015;11:494–503.e3.
Kim JP, Seo SW, Shin HY, Ye BS, Yang JJ, Kim C, et al. Effects of education on aging-related cortical thinning among cognitively normal individuals. Neurology. 2015;85:806–12.
Kim HJ, Yang JJ, Kwon H, Kim C, Lee JM, Chun P, et al. Relative impact of amyloid-beta, lacunes, and downstream imaging markers on cognitive trajectories. Brain. 2016;139:2516–27.
Kim HJ, Park S, Cho H, Jang YK, San Lee J, Jang H, et al. Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol 2018;75:999–1007.
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9.
Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid-beta PET with florbetaben (F-18) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–35.
Kim HJ, Park JY, Seo SW, Jung YH, Kim Y, Jang H, et al. Cortical atrophy pattern-based subtyping predicts prognosis of amnestic MCI: an individual-level analysis. Neurobiol Aging. 2019;74:38–45.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Jeon S, Yoon U, Park J-S, Seo SW, Kim J-H, Kim ST, et al. Fully automated pipeline for quantification and localization of white matter hyperintensity in brain magnetic resonance image. Int J Imaging Syst Technol. 2011;21:193–200.
Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–82.
Van Essen DC. A population-average, landmark-and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–62.
Sepulcre J, Schultz AP, Sabuncu M, Gomez-Isla T, Chhatwal J, Becker A, et al. In vivo tau, amyloid, and gray matter profiles in the aging brain. J Neurosci. 2016;36:7364–74.
Kang Y, Na DL. Seoul Neuropsychological Screening Battery (SNSB). Human Brain Research & Consulting Co., Seoul; 2003.
Ye BS, Seo SW, Cho H, Kim SY, Lee JS, Kim EJ, et al. Effects of education on the progression of early- versus late-stage mild cognitive impairment. Int Psychogeriatr. 2013;25:597–606.
Seo SW, Im K, Lee JM, Kim YH, Kim ST, Kim SY, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. Neuroimage. 2007;36:289–97.
Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91.
Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205.
Kwak K, Yoon U, Lee D-K, Kim GH, Seo SW, Na DL, et al. Fully-automated approach to hippocampus segmentation using a graph-cuts algorithm combined with atlas-based segmentation and morphological opening. Magn Reson Imaging. 2013;31:1190–6.
Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448–63.
Park JH, Seo SW, Kim C, Kim SH, Kim GH, Kim ST, et al. Effects of cerebrovascular disease and amyloid beta burden on cognition in subjects with subcortical vascular cognitive impairment. Neurobiol Aging. 2014;35:254–60.
Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–9.
Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, et al. Cerebrospinal fluid markers for Alzheimer’s disease evaluated after acute ischemic stroke. J Alzheimers Dis. 2000;2:199–206.
Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer’s disease: findings from neuropathological and neuroimaging studies. J Alzheimers Dis. 2014;42:S421–S9.
Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, et al. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–23.
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11.
Kim HJ, Cho H, Werring DJ, Jang YK, Kim YJ, Lee JS, et al. 18F-AV-1451 PET imaging in three patients with probable cerebral amyloid angiopathy. J Alzheimers Dis. 2017;57:711–6.
Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–65.
Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15:1044–53.
van Rossum IA, Vos SJ, Burns L, Knol DL, Scheltens P, Soininen H, et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology. 2012;79:1809–16.
Funding
This research was supported by funds (2018-ER6202-01) from Research of Korea Centers for Disease Control and Prevention; the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1913844); NRF grant funded by the Korea government (2017R1A2B2005081)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Role of the funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Jang, H., Kim, H.J., Park, S. et al. Application of an amyloid and tau classification system in subcortical vascular cognitive impairment patients. Eur J Nucl Med Mol Imaging 47, 292–303 (2020). https://doi.org/10.1007/s00259-019-04498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04498-y