Abstract
Purpose
The aim of this study was to establish a risk-stratification model integrating posttreatment metabolic response using the Deauville score and the pretreatment National Comprehensive Cancer Network-International Prognostic Index (NCCN-IPI) in nodal PTCLs.
Methods
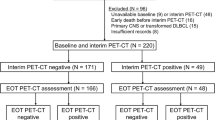
We retrospectively analysed 326 patients with newly diagnosed nodal PTCLs between January 2005 and June 2016 and both baseline and posttreatment PET/CT data. The final model was validated using an independent prospective cohort of 79 patients.
Results
Posttreatment Deauville score (1/2, 3, and 4/5) and the NCCN-IPI (low, low-intermediate, high-intermediate, and high) were independently associated with progression-free survival: for the Deauville score, the hazard ratios (HRs) were 1.00 vs. 2.16 (95% CI 1.47–3.18) vs. 7.86 (5.66–10.92), P < 0.001; and for the NCCN-IPI, the HRs were 1.00 vs. 2.31 (95% CI 1.20–4.41) vs. 4.42 (2.36–8.26) vs. 7.09 (3.57–14.06), P < 0.001. Based on these results, we developed a simplified three-group risk model comprising a low-risk group (low or low-intermediate NCCN-IPI with a posttreatment Deauville score of 1 or 2, or low NCCN-IPI with a Deauville score of 3), a high-risk group (high or high-intermediate NCCN-IPI with a Deauville score of 1/2 or 3, or low-intermediate NCCN-IPI with a Deauville score of 3), and a treatment failure group (Deauville score 4 or 5). This model was significantly associated with progression-free survival (5-year, 70.3%, 31.4%, and 4.7%; P < 0.001) and overall survival (5-year, 82.1%, 45.5%, and 14.7%; P < 0.001). Similar associations were also observed in the independent validation cohort.
Conclusion
The risk-stratification model integrating posttreatment Deauville score and pretreatment NCCN-IPI is a powerful tool for predicting treatment failure in patients with nodal PTCLs.



Similar content being viewed by others
Change history
17 September 2018
Unfortunately, the original version of this article contained several errors made during final step of article production. In the results section (fourth sentence) of the Abstract, the incomplete sentence,”, 31.4% in high-risk group and 4.7% in treatment failure group.
References
Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30. https://doi.org/10.1200/JCO.2008.16.4558.
Kim JM, Ko YH, Lee SS, Huh J, Kang CS, Kim CW, et al. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. Korean J Pathol. 2011;45(3):254–60. https://doi.org/10.4132/KoreanJPathol.2011.45.3.254.
Carson KR, Horwitz SM, Pinter-Brown LC, Rosen ST, Pro B, Hsi ED, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017;123(7):1174–83. https://doi.org/10.1002/cncr.30416.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. https://doi.org/10.1200/JCO.2013.54.8800.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–58. https://doi.org/10.1200/JCO.2013.53.5229.
El-Galaly TC, Pedersen MB, Hutchings M, Mylam KJ, Madsen J, Gang AO, et al. Utility of interim and end-of-treatment PET/CT in peripheral T-cell lymphomas: a review of 124 patients. Am J Hematol. 2015;90(11):975–80. https://doi.org/10.1002/ajh.24128.
Adams HJ, Kwee TC. 18F-FDG-PET is not a useful tool for end-of-treatment response evaluation in peripheral T-cell lymphomas. Nucl Med Commun. 2016;37(2):217. https://doi.org/10.1097/MNM.0000000000000437.
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–42. https://doi.org/10.1182/blood-2013-09-524108.
Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119(9):2066–73. https://doi.org/10.1182/blood-2011-06-359943.
Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [18F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J Clin Oncol. 2012;30(35):4317–22. https://doi.org/10.1200/JCO.2012.43.0934.
Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, et al. International validation study for interim PET in ABVD-treated, advanced-stage Hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54(5):683–90. https://doi.org/10.2967/jnumed.112.110890.
Cottereau AS, El-Galaly TC, Becker S, Broussais F, Petersen LJ, Bonnet C, et al. Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T-cell lymphoma patients. J Nucl Med. 2018;59(4):589–95. https://doi.org/10.2967/jnumed.117.193946.
Kim SJ, Choi JY, Hyun SH, Ki CS, Oh D, Ahn YC, et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. 2015;2(2):e66–74. https://doi.org/10.1016/S2352-3026(15)00002-2.
d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–9. https://doi.org/10.1200/JCO.2011.40.2719.
Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106–13. https://doi.org/10.1200/JCO.2008.17.4870.
Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99(6):1107–13. https://doi.org/10.3324/haematol.2013.103218.
Acknowledgments
We acknowledge the support of the Biomedical Research Institute, Chonbuk National University Hospital.
Funding
This study was supported by funding from the Biomedical Research Institute, Chonbuk National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
This study protocol was approved by the institutional review board (IRB) of each participating institution. Because this study was a retrospective analysis involving no more than minimal risk to the subjects, the IRB granted a waiver of written informed consent.
Additional information
The original version of this article was revised as it contains several errors made during the final step of article production.
Electronic supplementary material
ESM 1
(DOCX 341 kb)
Rights and permissions
About this article
Cite this article
Yhim, HY., Park, Y., Han, YH. et al. A risk stratification model for nodal peripheral T-cell lymphomas based on the NCCN-IPI and posttreatment Deauville score. Eur J Nucl Med Mol Imaging 45, 2274–2284 (2018). https://doi.org/10.1007/s00259-018-4093-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4093-1