Abstract
Purpose
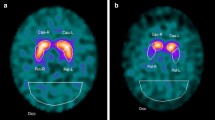
This work aimed to assess the potential of a set of features extracted from [123I]FP-CIT SPECT brain images to be used in the computer-aided “in vivo” confirmation of dopaminergic degeneration and therefore to assist clinical decision to diagnose Parkinson’s disease.
Methods
Seven features were computed from each brain hemisphere: five standard features related to uptake ratios on the striatum and two features related to the estimated volume and length of the striatal region with normal uptake. The features were tested on a dataset of 652 [123I]FP-CIT SPECT brain images from the Parkinson’s Progression Markers Initiative. The discrimination capacities of each feature individually and groups of features were assessed using three different machine learning techniques: support vector machines (SVM), k-nearest neighbors and logistic regression.
Results
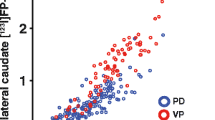
Cross-validation results based on SVM have shown that, individually, the features that generated the highest accuracies were the length of the striatal region (96.5%), the putaminal binding potential (95.4%) and the striatal binding potential (93.9%) with no statistically significant differences among them. The highest classification accuracy was obtained using all features simultaneously (accuracy 97.9%, sensitivity 98% and specificity 97.6%). Generally, slightly better results were obtained using the SVM with no statistically significant difference to the other classifiers for most of the features.
Conclusions
The length of the striatal region uptake is clinically useful and highly valuable to confirm dopaminergic degeneration “in vivo” as an aid to the diagnosis of Parkinson’s disease. It compares fairly well to the standard uptake ratio-based features, reaching, at least, similar accuracies and is easier to obtain automatically. Thus, we propose its day to day clinical use, jointly with the uptake ratio-based features, in the computer-aided diagnosis of dopaminergic degeneration in Parkinson’s disease.






Similar content being viewed by others
References
Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5:75–86. https://doi.org/10.1016/S1474-4422(05)70285-4.
Varrone A, Halldin C. New developments of dopaminergic imaging in Parkinson’s disease. Q J Nucl Med Mol Imaging. 2012;56:68–82.
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–25. https://doi.org/10.1001/archneur.61.6.919.
Kasanuki K, Iseki E, Ota K, Kondo D, Ichimiya Y, Sato K, et al. 123I-FP-CIT SPECT findings and its clinical relevance in prodromal dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2017;44:358–65. https://doi.org/10.1007/s00259-016-3466-6.
Cuberas-Borrós G, Lorenzo-Bosquet C, Aguadé-Bruix S, Hernández-Vara J, Pifarré-Montaner P, Miquel F, et al. Quantitative evaluation of striatal I-123-FP-CIT uptake in essential tremor and parkinsonism. Clin Nucl Med. 2011;36:991–6. https://doi.org/10.1097/RLU.0b013e3182291a7b.
Badiavas K, Molyvda E, Iakovou I, Tsolaki M, Psarrakos K, Karatzas N. SPECT imaging evaluation in movement disorders: far beyond visual assessment. Eur J Nucl Med Mol Imaging. 2011;38:764–73. https://doi.org/10.1007/s00259-010-1664-1.
Mirzaei S, Zakavi R, Rodrigues M, Schwarzgruber T, Brücke T, Bakala J, et al. Fully automated 3D basal ganglia activity measurement in dopamine transporter scintigraphy (Spectalyzer). Ann Nucl Med. 2010;24:295–300. https://doi.org/10.1007/s12149-010-0353-2.
Habraken JBA, Booij J, Slomka P, Sokole EB, van Royen EA. Quantification and visualization of defects of the functional dopaminergic system using an automatic algorithm. J Nucl Med. 1999;40:1091–7.
Jensen PS, Ziebell M, Skouboe G, Khalid U, Rd N, Thomsen G, et al. Validation of a method for accurate and highly reproducible quantification of brain dopamine transporter SPECT studies. J Nucl Med Technol. 2011;39:271–8. https://doi.org/10.2967/jnmt.111.090324.
Calvini P, Rodriguez G, Inguglia F, Mignone A, Guerra UP, Nobili F. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–53. https://doi.org/10.1007/s00259-006-0357-2.
Zubal IG, Early M, Yuan O, Jennings D, Marek K, Seibyl JP. Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson’s disease patients. J Nucl Med. 2007;48:857–64. https://doi.org/10.2967/jnumed.106.037432.
Koch W, Radau PE, Hamann C, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med. 2005;46:1109–18.
Morton RJ, Guy MJ, Clauss R, Hinton PJ, Marshall CA, Clarke EA. Comparison of different methods of DatSCAN quantification. Nucl Med Commun. 2005;26:1139–46.
Prashanth R, Roy SD, Mandal PK, Ghosh S. Automatic classification and prediction models for early Parkinson’s disease diagnosis from SPECT imaging. Expert Syst Appl. 2014;41:3333–42. https://doi.org/10.1016/j.eswa.2013.11.031.
Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629–35. https://doi.org/10.1016/j.pneurobio.2011.09.005.
Oliveira FPM, Tavares JMRS. Medical image registration: a review. Comput Method Biomech Biomed Engin. 2014;17:73–93. https://doi.org/10.1080/10255842.2012.670855.
Oliveira FPM, Faria DB, Costa DC, Tavares JMRS. A robust computational solution for automated quantification of a specific binding ratio based on [123I]FP-CIT SPECT images. Q J Nucl Med Mol Imaging. 2014;58:74–84.
Ibáñez L, Schroeder W, Ng L, Cates J, Consortium IS. The ITK software guide. Kitware, 2005. http://www.itk.org/.
Tossici-Bolt L, Hoffmann SMA, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9. https://doi.org/10.1007/s00259-006-0155-x.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cerebr Blood Flow Metab. 2007;27:1533–9. https://doi.org/10.1038/sj.jcbfm.9600493.
Schroeder W, Martin K, Lorensen B. Visualization toolkit: An object-oriented approach to 3D graphics. 4th ed. Clifton Park: Kitwarel; 2006.
Chang C-C, Lin C-J. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:1–27. https://doi.org/10.1145/1961189.1961199.
Nobili F, Naseri M, Carli FD, Asenbaum S, Booij J, Darcourt J, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2013;40:565–73. https://doi.org/10.1007/s00259-012-2304-8.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40:213–27. https://doi.org/10.1007/s00259-012-2276-8.
Illán IA, Górriz JM, Ramírez J, Segovia F, Jiménez-Hoyuela JM, Lozano SJO. Automatic assistance to Parkinson’s disease diagnosis in DaTSCAN SPECT imaging. Med Phys. 2012;39:5971–80. https://doi.org/10.1118/1.4742055.
Oliveira FPM, Castelo-Branco M. Computer-aided diagnosis of Parkinson’s disease based on [123I]FP-CIT SPECT binding potential images, using the voxels-as-features approach and support vector machines. J Neural Eng. 2015;12:026008. https://doi.org/10.1088/1741-2560/12/2/026008.
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on this study, visit the www.ppmi-info.org website. PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid, Biogen Idec, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lundbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche and UCB.
Funding
This work was partially financially supported by the public projects with the references: CENTRO-07-ST24-FEDER-00205 - “From molecules to man: novel diagnostic imaging tools in neurological and psychiatric disorders”; PTDC/BBB-BMD/3088/2012, financially supported by Fundação para a Ciência e a Tecnologia (FCT) in Portugal; and NORTE-01-0145-FEDER-000022 - SciTech - Science and Technology for Competitive and Sustainable Industries, co-financed by “Programa Operacional Regional do Norte” (NORTE2020), through “Fundo Europeu de Desenvolvimento Regional” (FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
We declare that all human data studies have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Oliveira, F.P.M., Faria, D.B., Costa, D.C. et al. Extraction, selection and comparison of features for an effective automated computer-aided diagnosis of Parkinson’s disease based on [123I]FP-CIT SPECT images. Eur J Nucl Med Mol Imaging 45, 1052–1062 (2018). https://doi.org/10.1007/s00259-017-3918-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3918-7