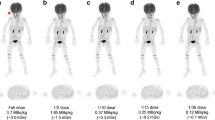
Abstract
Purpose
To explore the feasibility of reducing administered tracer activities and to assess optimal activities for combined 18F-FDG-PET/MRI in pediatric oncology.
Methods
30 18F-FDG-PET/MRI examinations were performed on 24 patients with known or suspected solid tumors (10 girls, 14 boys, age 12 ± 5.6 [1–18] years; PET scan duration: 4 min per bed position). Low-activity PET images were retrospectively simulated from the originally acquired data sets using randomized undersampling of list mode data. PET data of different simulated administered activities (0.25–2.5 MBq/kg body weight) were reconstructed with or without point spread function (PSF) modeling. Mean and maximum standardized uptake values (SUVmean and SUVmax) as well as SUV variation (SUVvar) were measured in physiologic organs and focal FDG-avid lesions. Detectability of organ structures and of focal 18F-FDG-avid lesions as well as the occurrence of false-positive PET lesions were assessed at different simulated tracer activities.
Results
Subjective image quality steadily declined with decreasing tracer activities. Compared to the originally acquired data sets, mean relative deviations of SUVmean and SUVmax were below 5 % at 18F-FDG activities of 1.5 MBq/kg or higher. Over 95 % of anatomic structures and all pathologic focal lesions were detectable at 1.5 MBq/kg 18F-FDG. Detectability of anatomic structures and focal lesions was significantly improved using PSF. No false-positive focal lesions were observed at tracer activities of 1 MBq/kg 18F-FDG or higher. Administration of 18F-FDG activities of 1.5 MBq/kg is, thus, feasible without obvious diagnostic shortcomings, which is equivalent to a dose reduction of more than 50 % compared to current recommendations.
Conclusion
Significant reduction in administered 18F-FDG tracer activities is feasible in pediatric oncologic PET/MRI. Appropriate activities of 18F-FDG or other tracers for specific clinical questions have to be further established in selected patient populations.




Similar content being viewed by others
References
Franzius C, Juergens KU. PET/CT in paediatric oncology: indications and pitfalls. Pediatr Radiol. 2009;39 Suppl 3:446–9.
Depas G, De Barsy C, Jerusalem G, et al. 18F-FDG PET in children with lymphomas. Eur J Nucl Med Mol Imaging. 2005;32(1):31–8.
Franzius C. FDG-PET/CT in pediatric solid tumors. Q J Nucl Med Mol Imaging. 2010;54(4):401–10.
Ricard F, Cimarelli S, Deshayes E, Mognetti T, Thiesse P, Giammarile F. Additional Benefit of F-18 FDG PET/CT in the staging and follow-up of pediatric rhabdomyosarcoma. Clin Nucl Med. 2011;36(8):672–7.
Volker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25(34):5435–41.
Chawla SC, Federman N, Zhang D, et al. Estimated cumulative radiation dose from PET/CT in children with malignancies: a 5-year retrospective review. Pediatr Radiol. 2010;40(5):681–6.
Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505.
Gatidis S, Schmidt H, Guckel B, et al. Comprehensive oncologic imaging in infants and preschool children with substantially reduced radiation exposure using combined simultaneous 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging. Investig Radiol. 2015.
Schafer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273(1):220–31.
Oehmigen M, Ziegler S, Jakoby BW, Georgi JC, Paulus DH, Quick HH. Radiotracer dose reduction in integrated PET/MR: implications from national electrical manufacturers association phantom studies. J Nucl Med. 2014;55(8):1361–7.
Hofmann M, Bezrukov I, Mantlik F, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med. 2011;52(9):1392–9.
Gatidis S, Wurslin C, Seith F, et al. Towards tracer dose reduction in PET studies: Simulation of dose reduction by retrospective randomized undersampling of list-mode data. Hell J Nucl Med. Mar 1 2016.
Stauss J, Franzius C, Pfluger T, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2008;35(8):1581–8.
Fahey FH, Bom HH, Chiti A, et al. Standardization of administered activities in pediatric nuclear medicine Part 2: a report of the first nuclear medicine global initiative. J Nucl Med. Mar 31 2016.
Fahey FH, Bom HH, Chiti A, et al. Standardization of administered activities in pediatric nuclear medicine: a report of the first nuclear medicine global initiative project, part 1-statement of the issue and a review of available resources. J Nucl Med. 2015;56(4):646–51.
Alessio AM, Sammer M, Phillips GS, Manchanda V, Mohr BC, Parisi MT. Evaluation of optimal acquisition duration or injected activity for pediatric 18F-FDG PET/CT. J Nucl Med. 2011;52(7):1028–34.
Aklan B, Oehmigen M, Beiderwellen K, et al. Impact of point-spread function (PSF) modeling on PET image quality in integrated PET/MR hybrid imaging. J Nucl Med. Oct 15 2015.
Gatidis S, Wurslin C, Seith F, et al. Towards tracer dose reduction in PET studies: Simulation of dose reduction by retrospective randomized undersampling of listmode data. HJNM. 2016.
Alessio AM, Kinahan PE, Manchanda V, Ghioni V, Aldape L, Parisi MT. Weight-based, low-dose pediatric whole-body PET/CT protocols. J Nucl Med. 2009;50(10):1570–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partially funded as a cooperation project with Siemens Healthcare (Erlangen, Germany).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was performed retrospectively on existing clinical imaging data. All legal guardians had given their written informed consent for the scientific analysis of acquired imaging data. Due to the retrospective nature of this study, formal approval was waived by the local ethics committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
2-year-old girl with metastatic teratoma. Series of simulated low-dose PET images (0.25–2.5 MBq/kg 18 F-FDG) and the originally acquired image reconstructed using PSF. The solitary pleural metastasis (arrow) is visible in all data sets. Delineation of anatomic details (e.g. thymus or bowel) steadily improves with increasing tracer activities. (BMP 15795 kb)
Supplementary Figure 2
16-year-old boy with Hodgkin’s lymphoma. Series of simulated low-dose PET images (0.25, 0.5, 1 and 1.5 MBq/kg 18 F-FDG) and the originally acquired image reconstructed using PSF. Lymph node involvement (arrows) and sacral bone marrow involvement (circle) become better detectable with increasing tracer activities. All lesions are detectable at 1.5 MBq/kg. (BMP 18646 kb)
Supplementary Figure 3
7-year-old girl with non-Hodgkin’s lymphoma. Comparison of PET images without (left) and with (right) PSF reconstruction at a simulated tracer activity of 1 MBq/kg. The use of PSF reconstruction results in a better general image quality and better delineation of the FDG-avid mass of the neck (arrow). (BMP 5303 kb)
Rights and permissions
About this article
Cite this article
Gatidis, S., Schmidt, H., la Fougère, C. et al. Defining optimal tracer activities in pediatric oncologic whole-body 18F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging 43, 2283–2289 (2016). https://doi.org/10.1007/s00259-016-3503-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3503-5