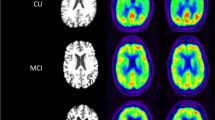
Abstract
Purpose
[18F]FDG is a commonly used neuronal injury biomarker for early and differential diagnosis of dementia. Typically, the blood supply to the brain is closely coupled to glucose consumption. Early uptake of the Aβ tracer [11C]PiB on PET images is mainly determined by cerebral blood flow and shows a high correlation with [18F]FDG uptake. Uptake data for 18F-labelled Aβ PET tracers are, however, scarce. We investigated the value of early PET images using the novel Aβ tracer [18F]FBB in the diagnosis of Alzhimers disease (AD).
Methods
This retrospective analysis included 22 patients with MCI or dementia who underwent dual time-point PET imaging with either [11C]PiB (11 patients) or [18F]FBB (11 patients) in routine clinical practice. Images were acquired 1 – 9 min after administration of both tracers and 40 – 70 min and 90 – 110 min after administration of [11C]PiB and [18F]FBB, respectively. The patients also underwent [18F]FDG brain PET imaging. PET data were analysed visually and semiquantitatively. Associations between early Aβ tracer uptake and dementia as well as brain atrophy were investigated.
Results
Regional visual scores of early Aβ tracer and [18F]FDG PET images were significantly correlated (Spearman’s ρ = 0.780, P < 0.001). Global brain visual analysis revealed identical results between early Aβ tracer and [18F]FDG PET images. In a VOI-based analysis, the early Aβ tracer data correlated significantly with the [18F]FDG data (r = 0.779, P < 0.001), but there were no differences between [18F]FBB and [11C]PiB. Cortical SUVRs in regions typically affected in AD on early Aβ tracer and [18F]FDG PET images were correlated with MMSE scores (ρ = 0.458, P = 0.032, and ρ = 0.456, P = 0.033, respectively). A voxel-wise group-based search for areas with relatively higher tracer uptake on early Aβ tracer PET images compared with [18F]FDG PET images revealed a small cluster in the midbrain/pons; no significant clusters were found for the opposite comparison.
Conclusion
Early [18F]FBB and [11C]PiB PET brain images are similar to [18F]FDG PET images in AD patients, and these tracers could potentially be used as biomarkers in place of [18F]FDG. Thus, Aβ tracer PET imaging has the potential to provide biomarker information on AD pathology and neuronal injury. The potential of this approach for supporting the diagnosis of AD needs to be confirmed in prospective studies in larger cohorts.



Similar content being viewed by others
References
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29.
Tiepolt S, Barthel H, Butzke D, Hesse S, Patt M, Gertz H, et al. Influence of scan duration on the accuracy of β-amyloid PET with florbetaben in patients with Alzheimer’s disease and healthy volunteers. Eur J Nucl Med Mol Imaging. 2013;40(2):238–44.
Barthel H, Seibyl J, Sabri O. The role of positron emission tomography imaging in understanding Alzheimer’s disease [ENG]. Expert review of neurotherapeutics. Expert Rev Neurother. 2015;15(4):395–406.
Schroeter ML, Neumann J. Combined imaging markers dissociate Alzheimer’s disease and frontotemporal lobar degeneration – an ALE meta-analysis. Front Aging Neurosci. 2011;3:10. doi:10.3389/fnagi.2011.00010.
Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer’s disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47(4):1196–206. doi:10.1016/j.neuroimage.2009.05.037.
Meyer PT, Hellwig S, Amtage F, Rottenburger C, Sahm U, Reuland P, et al. Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. J Nucl Med. 2011;52(3):393–400.
Becker GA, Ichise M, Barthel H, Luthardt J, Patt M, Seese A, et al. PET quantification of 18F-florbetaben binding to β-amyloid deposits in human brains. J Nucl Med. 2013;54(5):723–31.
Rostomian AH, Madison C, Rabinovici GD, Jagust WJ. Early 11C-PIB frames and 18F-FDG PET measures are comparable: a study validated in a cohort of AD and FTLD patients. J Nucl Med. 2011;52(2):173–9.
Forsberg A, Engler H, Blomquist G, Långström B, Nordberg A. The use of PIB-PET as a dual pathological and functional biomarker in AD. Biochim Biophys Acta. 2012;1822(3):380–5.
Hsiao I, Huang C, Hsieh C, Hsu W, Wey S, Yen T, et al. Correlation of early-phase 18F-florbetapir (AV-45/Amyvid) PET images to FDG images: preliminary studies. Eur J Nucl Med Mol Imaging. 2012;39(4):613–20.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44.
Werner P, Barthel H, Drzezga A, Sabri O. Current status and future role of brain PET/MRI in clinical and research settings. Eur J Nucl Med Mol Imaging. 2015;42(3):512–26.
Barthel H, Gertz H, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10(5):424–35.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–47.
Suotunen T, Hirvonen J, Immonen-Räihä P, Aalto S, Lisinen I, Arponen E, et al. Visual assessment of [(11)C]PIB PET in patients with cognitive impairment. Eur J Nucl Med Mol Imaging. 2010;37(6):1141–7.
Barthel H, Luthardt J, Becker G, Patt M, Hammerstein E, Hartwig K, et al. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011;38(9):1702–14.
Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer disease: phase 3 study. Alzheimers Dement. 2015;11(8):964–74. doi:10.1016/j.jalz.2015.02.004.
Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia – a meta-analysis. Neurobiol Aging. 2008;29(3):418–26. doi:10.1016/j.neurobiolaging.2006.10.023.
Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49(2):1490–5. doi:10.1016/j.neuroimage.2009.09.017.
Villemagne VL, Mulligan RS, Pejoska S, Ong K, Jones G, O’Keefe G, et al. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39(6):983–9.
Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55(10):967–72.
Blomquist G, Engler H, Nordberg A, Ringheim A, Wall A, Forsberg A, et al. Unidirectional Influx and net accumulation of PIB. Open Neuroimag J. 2008;2:114–25.
Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–28. doi:10.1016/S1474-4422(09)70299-6.
Arlt S, Brassen S, Jahn H, Wilke F, Eichenlaub M, Apostolova I, et al. Association between FDG uptake, CSF biomarkers and cognitive performance in patients with probable Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36(7):1090–100.
Kadir A, Almkvist O, Forsberg A, Wall A, Engler H, Långström B, et al. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer’s disease. Neurobiol Aging. 2012;33(1):98.e1–14.
Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. J Nucl Med. 2013;54:476–90.
Booij J, Arbizu J, Darcourt J, Hesse S, Nobili F, Payoux P, et al. Appropriate use criteria for amyloid PET imaging cannot replace guidelines: on behalf of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging. 2013;40(7):1122–5.
Acknowledgments
We thank all patients who took part in this trial. We also acknowledge the excellent support of the cyclotron, radiochemistry, and PET teams of the University of Leipzig, Department of Nuclear Medicine. Special thanks to Martin Wehner for his support in preparing the PET data analysis.
Assistance with editing the manuscript was provided by Michelle Thorpe (Bioscript Medical, London, UK) and funded by Piramal Imaging S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
H.B. and O.S. receive consultant or speaker honoraria from Piramal Imaging.
S.H. has received travel grants and honoraria from General Electric (GE) Healthcare and Bayer Schering Pharma. S.H. is in part funded by a grant from the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01E01001 (http://www.bmbf.de).
M.L.S. has been supported by LIFE – Leipzig Research Center for Civilization Diseases at the University. LIFE is funded by the European Union, by the European Regional Development Fund (ERFD) and by the Free State of Saxony within the framework of the Excellence Initiative. M.L.S. is also supported by the German Federal Ministry of Education and Research (BMBF; German FTLD consortium – grant no. FKZ 01GI1007A), and by the Parkinson’s Disease Foundation (grant no. PDF-IRG-1307).
M.P. received a speaker’s fee from Roche, holds patents in cooperation with Piramal and ABX and received research grants from Navidea and Piramal.
The other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tiepolt, S., Hesse, S., Patt, M. et al. Early [18F]florbetaben and [11C]PiB PET images are a surrogate biomarker of neuronal injury in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43, 1700–1709 (2016). https://doi.org/10.1007/s00259-016-3353-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3353-1