Abstract
Purpose
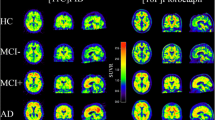
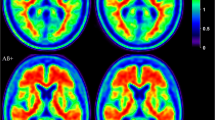
Preclinical, or asymptomatic, Alzheimer’s disease (AD) refers to the presence of positive AD biomarkers in the absence of cognitive deficits. This research concept is being applied to define target populations for clinical drug development. In a prospective community-recruited cohort of cognitively intact older adults, we compared two amyloid imaging markers within subjects: 18F-flutemetamol and 11C-Pittsburgh compound B (PIB).
Methods
In 32 community-recruited cognitively intact older adults aged between 65 and 80 years, we determined the concordance between binary classification based on 18F-flutemetamol versus 11C-PIB according to semiquantitative assessment (standardized uptake value ratio in composite cortical volume, SUVRcomp) and, alternatively, according to visual reads. We also determined the correlation between 18F-flutemetamol and 11C-PIB SUVR and evaluated how this was affected by the reference region chosen (cerebellar grey matter versus pons) and the use of partial volume correction (PVC) in this population.
Results
Binary classification based on semiquantitative assessment was concordant between 18F-flutemetamol and 11C-PIB in 94 % of cases. Concordance of blinded binary visual reads between tracers was 84 %. The Spearman correlation between 18F-flutemetamol and 11C-PIB SUVRcomp with cerebellar grey matter as reference region was 0.84, with a slope of 0.98. Correlations in neocortical regions were significantly lower with the pons as reference region. PVC improved the correlation in striatum and medial temporal cortex.
Conclusion
For the definition of preclinical AD based on 18F-flutemetamol, concordance with 11C-PIB was highest using semiquantitative assessment with cerebellar grey matter as reference region.






Similar content being viewed by others
References
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:280–92.
Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010;9:1118–27.
Knopman DS, Jack JC, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–82.
Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–65.
Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, et al. Whole-body biodistribution and radiation dosimetry of 18F-GE067: a radioligand for in vivo brain amyloid imaging. J Nucl Med 2009;50:818–22.
Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med 2009;50:1251–9.
Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 2008;7:129–35.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir F18). J Nucl Med 2010;51:913–20.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–19.
Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010;68:319–29.
Hatashita S, Yamasaki H, Suzuki Y, Tanaka K, Wakebe D, Hayakawa H. [18F]Flutemetamol amyloid-beta PET imaging compared with [11C]PIB across the spectrum of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2014;41:290–300.
Jagust WJ. Amyloid imaging: coming to a PET scanner near you. Ann Neurol 2010;68:277–8.
Cselényi Z, Jönhagen ME, Forsberg A, Halldin C, Julin P, Schou M, et al. Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J Nucl Med 2012;53:415–24.
Vandenberghe R, Adamczuk K, Dupont P, Van Laere K, Chételat G. Amyloid PET in clinical practice: its place in the multidimensional space of Alzheimer’s disease. Neuroimage Clin 2013;2:497–511.
Rowe CC, Pejoska S, Mulligan RS, Jones G, Chan JG, Svensson S, et al. Head-to-head comparison of 11C-PiB and 18F-AZD4694 (NAV4694) for β-amyloid imaging in aging and dementia. J Nucl Med 2013;54:880–6.
Adamczuk K, De Weer AS, Nelissen N, Chen K, Sleegers K, Bettens K, et al. Polymorphism of brain derived neurotrophic factor influences β amyloid load in cognitively intact apolipoprotein E ε4 carriers. Neuroimage Clin 2013;2:512–20.
Adamczuk K, De Weer AS, Nelissen N, Dupont P, Sunaert S, Bettens K, et al. Functional changes in the language network in response to increased amyloid deposition in cognitively intact older adults. Cereb Cortex. 2014. doi:10.1093/cercor/bhu286.
Nelissen N, Vandenbulcke M, Fannes K, Verbruggen A, Peeters R, Dupont P, et al. Abeta amyloid deposition in the language system and how the brain responds. Brain 2007;130:2055–69.
Ahmad R, Goffin K, Van den Stock J, De Winter FL, Cleeren E, Bormans G, et al. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur Neuropsychopharmacol 2014;24:242–50.
Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 2014;55:1623–8.
Müller-Gärtner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12:571–83.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10.
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013;12:357–67.
Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous Sr MD, Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 2015;11:1–15.
Acknowledgements
We would like to thank the staff of Nuclear Medicine, Neurology, and Radiology at the University Hospitals Leuven. Special thanks to Carine Schildermans, Dorien Timmers, Kwinten Porters and Mieke Steukers for help with the study.
Compliance with ethical standards
ᅟ
Funding
This study was funded by the Foundation for Alzheimer Research SAO-FRMA (grant numbers 09013, 11020, 13007); Research Foundation Flanders (grant number G.0660.09); KU Leuven (grant numbers OT/08/056, OT/12/097); IWT VIND; IWT TGO BioAdapt AD; Belspo IAP (grant number P7/11); Research Foundation Flanders senior clinical investigator grant to R.V. and K.V.L.; Research Foundation Flanders doctoral fellowship to K.A; and KH is supported by the Research Fund KU Leuven (grant numbers OT/11/087 and CREA/14/023). 18F-Flutemetamol was provided by GE Healthcare free of charge for this academic investigator-driven trial.
Conflicts of interest
Katarzyna Adamczuk, Jolien Schaeverbeke, Natalie Nelissen, Veerle Neyens, Mathieu Vandenbulcke, Karolien Goffin, Johan Lilja, Kelly Hilven, Patrick Dupont and Koen Van Laere declare that they have no conflicts of interest. Rik Vandenberghe was the PI of the phase 1 and 2 18F-flutemetamol trials. UZ Leuven has had a clinical trial agreement for these trials with GE Healthcare. RV has a consultancy agreement with GE Healthcare. 18F-Flutemetamol was provided by GE Healthcare for the conduct of reported investigator-driven trial free of cost.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adamczuk, K., Schaeverbeke, J., Nelissen, N. et al. Amyloid imaging in cognitively normal older adults: comparison between 18F-flutemetamol and 11C-Pittsburgh compound B. Eur J Nucl Med Mol Imaging 43, 142–151 (2016). https://doi.org/10.1007/s00259-015-3156-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3156-9