Abstract
Purpose
Recently, a GGGGCC hexanucleotide repeat expansion in the C9ORF72 gene, located on chromosome 9p21 has been demonstrated to be the commonest cause of familial amyotrophic lateral sclerosis (ALS) and to account for 5 to 10 % of apparently sporadic ALS. Relatively little is known about the brain metabolism profile of patients carrying the expansion. Our aim was to identify the [18F]FDG PET profile in ALS patients with the C9ORF72 expansion (C9ORF72-ALS).
Methods
Fifteen C9ORF72-ALS patients were compared with 12 patients with ALS and comorbid frontotemporal dementia (FTD) without the C9ORF72 expansion (ALS-FTD) and 30 cognitively normal patients with ALS without mutations of ALS-related genes (sALS). The three groups were then cross-matched to 40 neurologically normal controls. All patients underwent FDG PET within 4 months of diagnosis.
Results
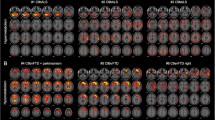
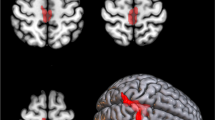
The C9ORF72-ALS patients compared with the sALS patients showed significant hypometabolism in the anterior and posterior cingulate cortex, insula, caudate and thalamus, the left frontal and superior temporal cortex, and hypermetabolism in the midbrain, bilateral occipital cortex, globus pallidus and left inferior temporal cortex. The ALS-FTD patients compared with the sALS patients showed more limited hypometabolic areas, including the orbitofrontal, prefrontal, anterior cingulate and insular cortex, and hypermetabolic areas, including the bilateral occipital cortex, the left precentral and postcentral cortex and superior temporal gyrus. The C9ORF72-ALS patients compared with the ALS-FTD patients showed hypometabolism in the left temporal cortex.
Conclusion
ALS patients with the C9ORF72 hexanucleotide repeat expansion had a more widespread central nervous system involvement than ALS patients without genetic mutations, with or without comorbid FTD, consistent with their more severe clinical picture.



Similar content being viewed by others
References
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55.
Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9ORF72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11:232–40.
Chiò A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, et al. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain. 2012;135:784–93.
Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56.
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68.
Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, et al. Frequency of the C9ORF72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–30.
Sabatelli M, Conforti FL, Zollino M, Mora G, Monsurrò MR, Volanti P, et al. C9ORF72 hexanucleotide repeat expansions in the Italian sporadic ALS population. Neurobiol Aging. 1848;2012(33):e15–20.
Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82:196–203.
Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–83.
Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
Brown JA, Min J, Staropoli JF, Collin E, Bi S, Feng X, et al. SOD1, ANG, TARDBP and FUS mutations in amyotrophic lateral sclerosis: a United States clinical testing lab experience. Amyotroph Lateral Scler. 2012;13:217–22.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54.
Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2009;10:131–46.
Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosci Psychiatry. 2011;83:102–8.
Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe): professional manual. Lutz, Fla, Psychological Assessment Resources, 2001.
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15.
Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis. 2011;26:627–45.
Caroli A, Prestia A, Chen K, Ayutyanont N, Landau SM, Madison CM, et al. Summary metrics to assess Alzheimer disease-related hypometabolic pattern with 18F-FDG PET: head-to-head comparison. J Nucl Med. 2012;53:592–600.
Cistaro A, Valentini MC, Chiò A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39:251–9.
Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranuline and sporadics. Brain. 2012;135:794–806.
Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–50.
Bede P, Bokde ALW, Byrne S, Elamin M, McLaughlin RL, Kenna K, et al. A multiparametric MRI study of ALS stratified for the C9ORF72 genotype. Neurology. 2013;81:361–9.
Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–90.
Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012;135:751–64.
Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–17.
Fujimoto T, Takeuch K, Matsumoto T, Kamimura K, Hamada R, Nakamura K, et al. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psych Res Neuroimaging. 2007;154:49–58.
Patel NH, Vyas NS, Puri BK, Nijran KS, Al Nahhas A. Positron emission tomography in schizophrenia: a new perspective. J Nucl Med. 2010;51:511–20.
Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–96.
Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009;199:53–60.
Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, et al. Repeat expansion in C9ORF72 in Alzheimer’s disease. N Engl J Med. 2012;366:283–4.
Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, et al. C9ORF72 repeat expansions are a rare genetic cause of parkinsonism. Brain. 2013;136:385–91.
Turner MR, Cagnin A, Turkheimer FE, Turkheimer F, Miller CCJ, Shaw CE. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–9.
Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C]-(L)-deprenyl-D2-PET. J Neurol Sci. 2007;255:17–22.
Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9.
Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–66.
Pioro EP, Majors AW, Mitsumoto H, Nelson DR, Ng TC. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology. 1999;53:71–9.
Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50:1585–93.
Pagani M, Dessi B, Morbelli S, Brugnolo A, Salmaso D, Piccini A, et al. MCI patients declining and not-declining at mid-term follow-up: FDG-PET findings. Curr Alzheimer Res. 2010;7:287–94.
Alessandrini M, Pagani M, Napolitano B, Micarelli A, Bruno E, Chiaravalloti A, et al. Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS One. 2013;8(3):e57596.
Nobili F, Mazzei D, Dessi B, Morbelli S, Brugnolo A, Barbieri P, et al. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J Alzheimers Dis. 2010;22:993–1003.
Acknowledgments
We thank the patients and the neurologically normal controls for their collaboration in this study. This work was supported in part by Compagnia di San Paolo, Programma Neuroscienze 2008-2009 (to M. Consuelo Valentini and A. Calvo), Ministero della Salute (Ricerca Sanitaria Finalizzata, 2010, grant RF-2010-2309849) (to A. Chiò) European Community’s Health Seventh Framework Programme (FP7/2007-2013 under grant agreement 259867) (to A. Chiò); The Intramural Research Programmes of the National Institutes of Health (NIH); National Institute on Aging (Z01-AG000949-02) (to Bryan J. Traynor).
Disclosures
Angelina Cistaro reports no disclosures.
Marco Pagani reports no disclosures.
Anna Montuschi reports no disclosures.
Andrea Calvo has received research support from the Ministry of Health (Ricerca Finalizzata), Regione Piemonte (Ricerca Finalizzata) and Compagnia di San Paolo.
Cristina Moglia reports no disclosures.
Antonio Canosa reports no disclosures.
Gabriella Restagno has received research support from the Ministry of Health (Ricerca Finalizzata), and Regione Piemonte (Ricerca Finalizzata).
Maura Brunetti reports no disclosures.
Bryan J. Traynor has a patent pending on the diagnostic and therapeutic uses of the C9ORF72 hexanucleotide repeat expansion.
Flavio Nobili reports no disclosures.
Giovanna Carrara reports no disclosures.
Piercarlo Fania reports no disclosures.
Leonardo Lopiano has received research support from the Ministry of Health (Ricerca Finalizzata), and Regione Piemonte (Ricerca Finalizzata).
M. Consuelo Valentini reports no disclosures.
Adriano Chiò has received research support from the Ministry of Health (Ricerca Finalizzata), Regione Piemonte (Ricerca Finalizzata), and European Commission (7th Framework Program); he serves on the editorial advisory board of Amyotrophic Lateral Sclerosis; he serves on a scientific advisory board for Biogen Idec and Cytokinetics.
Author Contributions
Study concept and design: Cistaro, Pagani, Chiò. Acquisition of data: Montuschi, Calvo, Moglia, Canosa, Restagno, Brunetti, Traynor, Nobili, Carrara, Fania. Analysis and interpretation of data: Cistaro, Pagani, Traynor, Nobili, Chiò. Drafting of the manuscript: Pagani, Chiò. Critical revision of the manuscript for important intellectual content: Cistaro, Pagani, Montuschi, Calvo, Restagno, Traynor, Valentini, Chiò. Obtained funding: Chiò. Administrative, technical, and material support: Cistaro, Pagani, Montuschi, Calvo, Moglia, Canosa, Brunetti, Restagno, Brunetti, Traynor, Nobili, Carrara, Fania, Lopiano, Valentini, Chiò. Study supervision: Cistaro, Pagani, Restagno, Valentini, Lopiano, Chiò.
Adriano Chiò had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the submitted version of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Angelina Cistaro and Marco Pagani contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure E-1
(DOCX 104 kb)
Figure E-2
(DOCX 104 kb)
Figure E-3
(DOCX 101 kb)
Figure E-4
(DOCX 102 kb)
Figure E-5
(DOCX 100 kb)
Figure E-6
(DOCX 103 kb)
Figure E-7
(DOCX 102 kb)
Figure E-8
(DOCX 102 kb)
Figure E-9
(DOCX 100 kb)
Figure E-10
(DOCX 103 kb)
Figure E-11
(DOCX 96.4 kb)
Table E-1
(DOC 45.5 kb)
Table E-2
(DOC 55.5 kb)
Table E-3
(DOC 79.5 kb)
Table E-4
(DOC 56 kb)
Table E-5
(DOC 30.5 kb)
Table E-6
(DOC 124 kb)
Table E-7
(DOC 167 kb)
Rights and permissions
About this article
Cite this article
Cistaro, A., Pagani, M., Montuschi, A. et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging 41, 844–852 (2014). https://doi.org/10.1007/s00259-013-2667-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2667-5