Abstract
Purpose
We have reported on the development of a novel PET probe, 18F-2-tert-butyl-4-chloro-5-{6-[2-(2-fluoroethoxy)-ethoxy]-pyridin-3-ylmethoxy}-2H-pyridazin-3-one (18F-BCPP-EF), for quantitative imaging of mitochondrial complex 1 (MC-1) activity in the brain of the living rat. For clinical application in humans, translational research in the monkey was conducted.
Methods
PET measurements with 18F-BCPP-EF were performed in young and old monkeys (Macaca mulatta) in a conscious state with arterial blood sampling. The binding specificity of 18F-BCPP-EF was evaluated with rotenone, a specific MC-1 inhibitor, in young animals. The binding (total distribution volume, V T) of 18F-BCPP-EF was calculated using Logan graphical analysis, and one-tissue compartment model (1-TC) and two-tissue compartment model (2-TC) analyses using a metabolite-corrected plasma input function.
Results
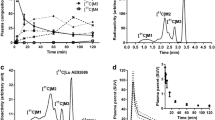
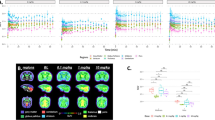
F-BCPP-EF was rapidly taken up into the brain just after intravenous injection, peaked between 10 and 20 min after injection, and was then gradually eliminated. The 2-TC analysis provided a better fit than the 1-TC analysis, and the V T values from the 2-TC analysis correlated well with those from the Logan plot. With predosing with rotenone, 18F-BCPP-EF showed a higher uptake peak in the brain, followed by more rapid elimination thereafter than in the vehicle condition, resulting in significant reductions in 2-TC V T values in all regions. In old animals, the kinetics of 18F-BCPP-EF were slightly slower with lower peak levels than in young animals, resulting age-related reductions in 18F-BCPP-EF binding in all brain regions.
Conclusion
The present study demonstrated that 18F-BCPP-EF may be a potential PET probe for quantitative imaging MC-1 activity in the living brain using PET.





Similar content being viewed by others
References
Meisenzahl EM, Schmitt GJ, Scheuerecker J, Möller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–45.
Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry. 2006;59:919–28.
Brooke DJ. Imaging approaches to Parkinson disease. J Nucl Med. 2010;51:596–609.
Maetzler W, Liepelt I, Berg D. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. 2009;8:1158–71.
Kadir A, Nordberg A. Target-specific PET probes for neurodegenerative disorders related to dementia. J Nucl Med. 2010;51:1418–30.
Neyer JH. Neuroimaging markers of cellular function in major depressive disorder: implications for therapeutics, personalized medicine, and prevention. Clin Pharmacol Ther. 2012;91:201–14.
Schroeter M, Dennin MA, Walberer M, Backes H, Neumaier B, Fink GR, et al. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J Cereb Blood Flow Metab. 2009;29:1216–25.
Fukumoto D, Hosoya T, Nishiyama S, Harada N, Iwata H, Yamamoto S, et al. Multiparametric assessment of acute and sub-acute ischemic neuronal damage: a small animal PET study with rat photochemically induced thrombosis (PIT) model. Synapse. 2011;65:207–14.
Winkeler A, Boisgard R, Martin A, Tavitian B. Radioisotopic imaging of neuroinflammation. J Nucl Med. 2010;51:1–4.
Jacobs AH, Tavitian B, INMiND Consortium. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32:1393–415.
Purohit A, Radeke H, Azure M, Hanson K, Benetti R, Su F, et al. Synthesis and biological evaluation of pyridazinone analogues as potential cardiac positron emission tomography tracers. J Med Chem. 2008;51:2954–70.
Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782–8.
Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49:630–6.
Fukumoto D, Nishiyama S, Harada N, Yamamoto S, Tsukada H. Detection of ischemic neuronal damage with [18F]BMS-747158-02, a mitochondrial complex-1 PET ligand: small animal PET study in rat brain. Synapse. 2012;66:909–17.
Harada N, Nishiyama S, Kanazawa M, Tsukada H. Development of novel PET probes, [18F]BCPP-EF, [18F]BCPP-BF, and [11C]BCPP-EM for mitochondrial complex 1 imaging in the living brain. J Label Compd Radiopharm. 2013;56:553–61.
Noda A, Takamatsu H, Minoshima S, Tsukada H, Nishimura S. Determination of kinetic rate constants for FDG and partition coefficient of water in conscious macaque and alterations in aging or anesthesia examined on parametric images with an anatomic standardization technique. J Cereb Blood Flow Metab. 2003;23:1441–7.
Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Cholinergic neuronal modulation alters dopamine D2 receptor availability in vivo by regulating receptor affinity induced by facilitated synaptic dopamine turnover? Positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci. 2000;20:7067–73.
Watanabe M, Okada H, Shimizu K, Omura T, Yoshikawa E, Kosugi T, et al. A high resolution animal PET scanner using compact PS-PMT detectors. IEEE Trans Nucl Sci. 1997;44:1277–82.
Jones EG, Stone JM, Karten HJ. High-resolution digital brain atlases: a Hubble telescope for the brain. Ann N Y Acad Sci. 2011;1225(S1):E147–59.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7.
Huang SH, Barrio J, Phelps M. Neuroreceptor assay with positron emission tomography; equilibrium versus dynamic approach. J Cereb Blood Flow Metab. 1986;6:515–21.
Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27.
Phelps ME. PET molecular imaging and its biological applications. New York: Springer; 2004. p. 125–216.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–23.
Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol Imaging Biol. 2003;5:376–89.
Carson RE, Kiesewetter DO, Jagoda E, Der MG, Herscovich P, Eckelman WC. Muscarinic cholinergic receptor measurements with [18F]FP-TZTP: control and competition studies. J Cereb Blood Flow Metab. 1998;18:1130–42.
Nishiyama S, Tsukada H, Sato K, Kakiuchi T, Ohba H, Harada N, et al. Evaluation of PET ligands (+)N-[11C]ethyl-3-piperidyl benzilate and (+)N-[11C]propyl-3-piperidyl benzilate for muscarinic cholinergic receptors: a PET study with microdialysis in comparison with (+)N-[11C]methyl-3-piperidyl benzilate in the conscious monkey brain. Synapse. 2001;40:159–69.
Koeppe RA, Frey KA, Mulholland GK, Kilbourn MR, Buck A, Lee KS, et al. [11C]Tropanyl benzilate binding to muscarinic cholinergic receptors: methodology and kinetic modeling alterations. J Cereb Blood Flow Metab. 1994;14:85–99.
Farde L, Eriksson L, Blomqvist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab. 1989;9:696–708.
Logan J, Volkow ND, Fowler JS, Wang G-J, Dewey SL, MacGregor R, et al. Effects of blood flow on [11C]raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab. 1994;14:995–1010.
Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–7.
Cadenas E, Boveris A, Ragan CI, Stoppani AOM. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180:248–57.
Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev. 1999;111:39–47.
Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86.
Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–8.
Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92:494–504.
Benzi G, Pastoris O, Marzatico F, Villa RF, Dagani F, Curti D. The mitochondrial electron transfer alteration as a factor involved in the brain aging. Neurobiol Age. 1992;13:361–8.
Acknowledgments
We gratefully acknowledge the technical assistance provided by Aiko Iwazaki, Shingo Nishiyama, and Shigeyuki Yamamoto.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukada, H., Ohba, H., Kanazawa, M. et al. Evaluation of 18F-BCPP-EF for mitochondrial complex 1 imaging in the brain of conscious monkeys using PET. Eur J Nucl Med Mol Imaging 41, 755–763 (2014). https://doi.org/10.1007/s00259-013-2628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2628-z