Abstract
Purpose
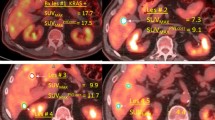
Standardized added metabolic activity (SAM) is a PET parameter for assessing the total metabolic load of malignant processes, avoiding partial volume effects and lesion segmentation. The potential role of this parameter in the assessment of response to chemotherapy and bevacizumab was tested in patients with metastatic colorectal cancer with potentially resectable liver metastases (mCRC).
Methods
18F-FDG PET/CT was performed in 18 mCRC patients with liver metastases before treatment and after five cycles of FOLFOX/FOLFIRI and bevacizumab. Of the 18 patients, 16 subsequently underwent resection of liver metastases. Baseline and follow-up SUVmax, and SAM as well as reduction in SUVmax (∆SUVmax) and SAM (∆SAM) of all liver metastases were correlated with morphological response, and progression-free and overall survival (PFS and OS).
Results
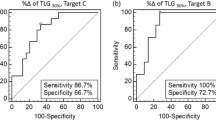
A significant reduction in metabolic activity of the liver metastases was seen after chemotherapy with a median ∆SUVmax of 25.3 % and ∆SAM of 94.5 % (p = 0.033 and 0.003). Median baseline SUVmax and SAM values were significantly different between morphological responders and nonresponders (3.8 vs. 7.2, p = 0.021; and 34 vs. 211, p = 0.002, respectively), but neither baseline PET parameters nor morphological response was correlated with PFS or OS. Follow-up SUVmax and SAM as well as ∆SAM were found to be prognostic factors. The median PFS and OS in the patient group with a high follow-up SUVmax were 10.4 months and 32 months, compared to a median PFS of 14.7 months and a median OS which had not been reached in the group with a low follow-up SUVmax (p = 0.01 and 0.003, respectively). The patient group with a high follow-up SAM and a low ∆SAM had a median PFS and OS of 9.4 months and 32 months, whereas the other group had a median PFS of 14.7 months and a median OS which had not been reached (p = 0.002 for both PFS and OS).
Conclusion
18F-FDG PET imaging is a useful tool to assess treatment response and predict clinical outcome in patients with mCRC who undergo chemotherapy before liver metastasectomy. Follow-up SUVmax, follow-up SAM and ∆SAM were found to be significant prognostic factors for PFS and OS.




Similar content being viewed by others
References
Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–6.
Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110–8. doi:10.1002/bjs.7032.
Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–99. doi:10.1038/sj.bjc.6603033.
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–76. doi:10.1245/ASO.2006.05.039.
Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–26. doi:10.1002/cncr.22448.
Nordlinger B, Van Cutsem E, Rougier P, Kohne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037–45. doi:10.1016/j.ejca.2007.07.017.
Chaudhury P, Hassanain M, Bouganim N, Salman A, Kavan P, Metrakos P. Perioperative chemotherapy with bevacizumab and liver resection for colorectal cancer liver metastasis. HPB (Oxford). 2010;12:37–42. doi:10.1111/j.1477-2574.2009.00119.x.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi:10.1056/NEJMoa032691.
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9. doi:10.1200/JCO.2010.33.5091.
Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–802. doi:10.1016/j.clinthera.2006.11.015.
Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012;14:e31–47. doi:10.1111/j.1463-1318.2011.02765.x.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Klinger M, Eipeldauer S, Hacker S, Herberger B, Tamandl D, Dorfmeister M, et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J Surg Oncol. 2009;35:515–20. doi:10.1016/j.ejso.2008.12.013.
Desar IM, van Herpen CM, van Laarhoven HW, Barentsz JO, Oyen WJ, van der Graaf WT. Beyond RECIST: molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev. 2009;35:309–21. doi:10.1016/j.ctrv.2008.12.001.
Sharma MR, Maitland ML, Ratain MJ. RECIST: no longer the sharpest tool in the oncology clinical trials toolbox – point. Cancer Res. 2012;72:5145–9. doi:10.1158/0008-5472.CAN-12-0058.
de Langen AJ, van den Boogaart V, Lubberink M, Backes WH, Marcus JT, van Tinteren H, et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J Nucl Med. 2011;52:48–55. doi:10.2967/jnumed.110.078261.
de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009;50 Suppl 1:43S–54S. doi:10.2967/jnumed.108.057224.
Vriens D, van Laarhoven HW, van Asten JJ, Krabbe PF, Visser EP, Heerschap A, et al. Chemotherapy response monitoring of colorectal liver metastases by dynamic Gd-DTPA-enhanced MRI perfusion parameters and 18F-FDG PET metabolic rate. J Nucl Med. 2009;50:1777–84. doi:10.2967/jnumed.109.064790.
Hendlisz A, Golfinopoulos V, Garcia C, Covas A, Emonts P, Ameye L, et al. Serial FDG-PET/CT for early outcome prediction in patients with metastatic colorectal cancer undergoing chemotherapy. Ann Oncol. 2012;23:1687–93. doi:10.1093/annonc/mdr554.
Visvikis D, Hatt M, Tixier F, Cheze Le Rest C. The age of reason for FDG PET image-derived indices. Eur J Nucl Med Mol Imaging. 2012;39:1670–2. doi:10.1007/s00259-012-2239-0.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71.
Mertens J, Dobbeleir A, Ham H, D’Asseler Y, Goethals I, Van de Wiele C. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2012;39:1441–8. doi:10.1007/s00259-012-2166-0.
Fleming JS, Tossici-Bolt L, Guy M, Kemp P. Comment on Mertens et al.: standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2013;40:788–9. doi:10.1007/s00259-013-2364-4.
Fleming JS, Bolt L, Stratford JS, Kemp PM. The specific uptake size index for quantifying radiopharmaceutical uptake. Phys Med Biol. 2004;49:N227–34.
Bogaerts J, Ford R, Sargent D, Schwartz LH, Rubinstein L, Lacombe D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–60.
Heijmen L, de Geus-Oei LF, de Wilt JH, Visvikis D, Hatt M, Visser EP, et al. Reproducibility of functional volume and activity concentration in 18F-FDG PET/CT of liver metastases in colorectal cancer. Eur J Nucl Med Mol Imaging. 2012;39:1858–67. doi:10.1007/s00259-012-2233-6.
Bystrom P, Berglund A, Garske U, Jacobsson H, Sundin A, Nygren P, et al. Early prediction of response to first-line chemotherapy by sequential [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with advanced colorectal cancer. Ann Oncol. 2009;20:1057–61. doi:10.1093/annonc/mdn744.
Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48:771–5. doi:10.2967/jnumed.106.037291.
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204.
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Ruhl A, Irngartinger G, Stremmel W, et al. Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med. 2004;45:1480–7.
de Geus-Oei LF, van Laarhoven HW, Visser EP, Hermsen R, van Hoorn BA, Kamm YJ, et al. Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol. 2008;19:348–52.
Dimitrakopoulou-Strauss A, Strauss LG, Rudi J. PET-FDG as predictor of therapy response in patients with colorectal carcinoma. Q J Nucl Med. 2003;47:8–13.
Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med. 2007;48 Suppl 1:36S–44S.
Hatt M, Cheze Le Rest C, Albarghach N, Pradier O, Visvikis D. PET functional volume delineation: a robustness and repeatability study. Eur J Nucl Med Mol Imaging. 2011;38:663–72. doi:10.1007/s00259-010-1688-6.
Acknowledgments
This work was supported by an unrestricted educational grant from Roche and by the Belgian National Cancer Plan (NKP29/026).
Conflicts of interest
M.P. received an educational grant from Roche. The other authors disclose no conflicts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mertens, J., De Bruyne, S., Van Damme, N. et al. Standardized added metabolic activity (SAM) IN 18F-FDG PET assessment of treatment response in colorectal liver metastases. Eur J Nucl Med Mol Imaging 40, 1214–1222 (2013). https://doi.org/10.1007/s00259-013-2421-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2421-z