Abstract
Purpose
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive method to excite neurons in the brain. However, the underlying mechanism of its therapeutic effects in stroke remains unclear. The aim of this study was to investigate the neuroprotective effect of high-frequency rTMS in a rat model of transient cerebral ischaemia using positron emission tomography (PET).
Methods
Sprague-Dawley rats (n=30) were anaesthetized with chloral hydrate and subjected to 90 min of intraluminal middle cerebral artery occlusion (MCAO) with subsequent reperfusion in three groups: control (n=10), rTMS (n=10), or sham-rTMS groups (n=10). In the rTMS group, rTMS was given 1 h after ischaemia and every 24 h for 7 days after MCAO. In all three groups, small-animal PET (microPET) imaging with 18F-FDG was used to evaluate brain glucose metabolism. Apoptotic molecules were measured in the infarct margin using immunohistochemical staining.
Results
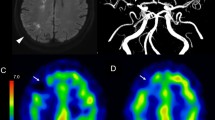
The neurological scores of the rats in the rTMS group were higher than in those of the control group over the whole 7-day observation period. The total, cortical and striatal infarct volumes were significantly less in the rTMS group than in the control group, as measured by 2,3,5-triphenyltetrazolium chloride staining. 18F-FDG microPET images showed significantly higher standardized uptake values in the cortex and striatum in the rTMS group than in the control group in the affected hemisphere. The number of cells positive for caspase-3 was significantly lower in the rTMS group than in the control group, while the Bcl-2/Bax ratio was significantly higher in the rTMS group than in the control group.
Conclusion
rTMS therapy increased glucose metabolism and inhibited apoptosis in the ischaemic hemisphere. 18F-FDG PET could be used to monitor rTMS therapy in transient cerebral ischaemia in animal studies and in future clinical trials.






Similar content being viewed by others
References
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7.
Maeda F, Keenan JP, Tormos JM, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 2000;111:800–5.
Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 2005;64:1802–4.
Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 2004;115:1519–26.
Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 2006;37:1471–6.
Fujiki M, Kobayashi H, Abe T, et al. Repetitive transcranial magnetic stimulation for protection against delayed neuronal death induced by transient ischemia. J Neurosurg 2003;99:1063–9.
Ogiue-Ikeda M, Kawato S, Ueno S. Acquisition of ischemic tolerance by repetitive transcranial magnetic stimulation in the rat hippocampus. Brain Res 2005;1037:7–11.
Kluk RM, Bossy-Wetzel E, Green DR, et al. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997;275:1132–6.
Ferrer I, Friguls B, Dalfó E, et al. Caspase-dependent and caspase-independent signalling of apoptosis in the penumbra following middle cerebral artery occlusion in the adult rat. Neuropathol Appl Neurobiol 2003;29:472–81.
Ouyang YB, Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium 2004;36:303–11.
Plesni N. Role of mitochondrial proteins for neuronal cell death after focal cerebral ischemia. Acta Neurochir Suppl 2004;89:15–9.
Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 1998;18:3659–68.
Chugani HT, Hovda DA, Villablanca JR, et al. Metabolic maturation of the brain: a study of local cerebral glucose utilization in the developing cat. J Cereb Blood Flow Metab 1991;11:35–47.
Bruehl C, Witte OW. Cellular activity underlying altered brain metabolism during focal epileptic activity. Ann Neurol 1995;38:414–20.
Kimbrell TA, Little JT, Dunn RT, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry 1999;46:1603–13.
Kimbrell TA, Dunn RZ, George MS, et al. Left prefrontal-repetitive transcranial magnetic stimulation (rTMS) and regional cerebral glucose metabolism in normal volunteers. Psychiatry Res 2002;115:101–3.
Speer AM, Kimbrell TA, Wassermann EM, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000;48:1133–41.
Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91.
Luft AR, Kaelin-Lang A, Hauser TK, et al. Transcranial magnetic stimulation in the rat. Exp Brain Res 2001;140:112–21.
Linden RD, Zhang YP, Burke DA, et al. Magnetic motor evoked potential monitoring in the rat. J Neurosurg 1999;91:205–10.
Lou M, Zhang H, Wang J, et al. Hyperbaric oxygen treatment attenuated the decrease in regional glucose metabolism of rats subjected to focal cerebral ischemia: a high resolution positron emission tomography study. Neuroscience 2007;46:555–61.
Zhang H, Zheng XS, Yang XF, et al. 11C-NMSP/18F-FDG microPET to monitor neural stem cell transplantation in a rat model of traumatic brain injury. Eur J Nucl Med Mol Imaging 2008;35:1699–708.
Wan H, Zhu H, Tian M, et al. Protective effect of chuanxiongzine-puerarin in a rat model of transient middle cerebral artery occlusion-induced focal cerebral ischemia. Nucl Med Commun 2008;29:1113–22.
Gao F, Guo Y, Zhang H, et al. Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: an FDG-microPET study in rats. Neurobiol Dis 2009;34:477–83.
Guo Y, Gao F, Wang S. et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 2009;162:972–9.
Matsumura A, Mizokawa S, Tanaka M, et al. Assessment of microPET performance in analyzing the rat brain under different types of anesthesia: comparison between quantitative data obtained with microPET and ex vivo autoradiography. Neuroimage 2003;20:2040–50.
Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke 1995;26:627–34.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998.
Xu J, Culman J, Blume A, et al. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke 2003;34:1287–92.
Pulsinelli W. Pathophysiology of acute cerebral ischemia. Lancet. 1992;359:533–6.
Cherry SR, Gambhir SS. Use of positron emission tomography in animal research. ILAR J 2001;42:219–32.
Lubec B, Chiappe-Gutierrez M, Hoeger H, et al. Glucose transporters, hexokinase, and phosphofructokinase in brain of rats with perinatal asphyxia. Pediatr Res 2000;47:84–8.
Li P, Nijhawan D, Budihardio I. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91:479–89.
Hu XL, Olsson T, Johansson IM. Dynamic changes of the anti- and pro-apoptotic proteins Bcl-w, Bcl-2, and Bax with Smac/Diablo mitochondrial release after photothrombotic ring stroke in rats. Eur J Neurosci 2004;20:1177–88.
Xing B, Chen H, Zhang M, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke 2008;39:2362–9.
Müller MB, Toschi N, Kresse AE, et al. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology 2000;23:205–15.
Kokaia Z, Zhao Q, Kokaia M, et al. Regulation of BDNF gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol 1995;136:73–88.
Schmid-Elsaesser RZS, Hungerhuber E, Baethmann A, et al. Critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 1998;29:2162–70.
Acknowledgments
This study was supported by grants from the National Science Foundation of China (NSFC) (nos. 30672396, 30600194), a key project grant from the Ministry of Science and Technology of China (MOST; no. 2006DFB32940), and a project grant from the Science and Technology Department of Zhejiang Province (no. 2009C33134).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, F., Wang, S., Guo, Y. et al. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging 37, 954–961 (2010). https://doi.org/10.1007/s00259-009-1342-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1342-3