Abstract
Purpose
The aims were to determine if the maximum standardized uptake value (SUVmax) of the primary tumor as determined by preoperative 18F-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) is an independent predictor of overall survival and to assess its prognostic value after stratification according to pathological staging.
Methods
A retrospective clinicopathologic review of 363 patients who had a preoperative 18F-FDG PET done before undergoing attempted curative resection for early-stage (I & II) non-small cell lung cancer (NSCLC) was performed. Patients who had received any adjuvant or neoadjuvant chemotherapy or radiation therapy were excluded. The primary outcome measure was duration of overall survival. Receiver-operating characteristic (ROC) curves were plotted to find out the optimal cutoff values of SUVmax yielding the maximal sensitivity plus specificity for predicting the overall survival. Survival curves stratified by median SUVmax and optimal cutoff SUVmax were estimated by the Kaplan-Meier method and statistical differences were assessed using the log-rank test. Multivariate proportional hazards (Cox) regression analyses were applied to test the SUVmax’s independency of other prognostic factors for the prediction of overall survival.
Results
The median duration of follow-up was 981 days (2.7 years). The median SUVmax was 5.9 for all subjects, 4.5 for stage IA, 8.4 for stage IB, and 10.9 for stage IIB. The optimal cutoff SUVmax was 8.2 for all subjects. No optimal cutoff could be established for specific stages. In univariate analyses, each doubling of SUVmax [i.e., each log (base 2) unit increase in SUVmax] was associated with a 1.28-fold [95% confidence interval (CI): 1.03–1.59, p = 0.029] increase in hazard of death. Univariate analyses did not show any significant difference in survival by SUVmax when data were stratified according to pathological stage (p = 0.119, p = 0.818, and p = 0.882 for stages IA, IB, and IIB, respectively). Multivariate analyses demonstrated that SUVmax was not an independent predictor of overall survival (p > 0.05).
Conclusion
Each doubling of SUVmax as determined by preoperative PET is associated with a 1.28-fold increase in hazard of death in early-stage (I & II) NSCLC. Preoperative SUVmax is not an independent predictor of overall survival.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
18F-Fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) has been demonstrated to be very useful in the diagnosis and staging of non-small cell lung cancers (NSCLC) [1, 2]. As a result there has been increasing interest in the prognostic utility of the preoperative standardized uptake value (SUV) of the primary tumor. Many prior studies have investigated this issue [3–16]. Several prior studies have reported an association between higher maximum SUV (SUVmax) and poor prognosis, but some recent studies have failed to find any independent correlation between them. To elucidate the prognostic significance of preoperative SUVmax of the primary tumor in early-stage (I & II) NSCLC, a retrospective review of 363 consecutive patients who had a preoperative 18F-FDG PET done before undergoing attempted curative resection for early-stage (I & II) NSCLC was performed.
Materials and methods
Approval of human data exemption was obtained from the Institutional Review Board for this Health Insurance Portability and Accountability Act (HIPAA) compliant study.
Objectives
The aims were to determine if SUVmax as measured by preoperative 18F-FDG PET is an independent predictor of survival and to determine if SUVmax as measured by preoperative 18F-FDG PET is of prognostic value after stratification according to pTNM staging.
Study population
Subjects were identified through the institutional tumor registry.
Patients were included in the study if they had a preoperative 18F-FDG PET done from 5 February 1999 through 21 March 2007 before undergoing attempted curative resection for pathologically documented stage I and II NSCLC and histological diagnosis was available.
Patients were excluded from the study if they had received any adjuvant or neoadjuvant chemotherapy or radiation therapy, had any prior history of lung cancer, or if SUVmax was not available due to the non-availability of SUVmax from the reports and the non-availability of PET images.
PET
Scans performed since July 2004 were obtained by a dedicated 16-slice whole-body PET/CT scanner (GE Discovery DST, GE Medical Systems, Milwaukee, WI, USA). All patients with 4-h fasting before the examination received an average of 560 MBq 18F-FDG intravenous injections. PET images were obtained 1 h after injection. The PET images were obtained at each bed position for 3 min with six to eight bed positions to cover the entire body. The PET images were obtained using a two-dimensional high-sensitivity mode with an axial field of view of 15 cm in a 256 × 256 matrix. A 3-slice overlap was utilized between the bed positions. The PET images were reconstructed iteratively on a 128 × 128 matrix using an ordered subsets expectation maximization algorithm for 30 subsets and two iterations, with a 7.0-mm post-reconstruction filter. In-plane resolution of 6.2 mm and axial resolution of 5.0 mm was obtained. Concomitant CT data were used for attenuation correction of all PET images in the quantitative analysis of SUV. The CT component of image acquisition used the following imaging parameters: 140 kVp, 120–200 mA, 0.8 s per CT rotation, pitch 1.75:1, detector configuration of 16 × 1.25 mm, and 3-mm slice thickness with oral contrast only.
PET and CT images were merged (fusion analysis) for functional and anatomic correlation. CT/PET images were displayed on AW/Xeleris and Medview workstations (General Electric Medical Systems, Milwaukee, WI, USA and Medimage, Ann Arbor, MI, USA). Scans performed before July 2004 were obtained on a dedicated whole-body PET only scanner (Advance, General Electric Medical Systems, Milwaukee, WI, USA) 1 h after injection of 18F-FDG (370 MBq, on average) and after the patients had fasted about 4 h. PET images were reconstructed using an iterative reconstruction algorithm with segmented attenuation correction. All PET data were visually examined and compared to the patient’s recent CT. The decision was finally made by correlating PET with CT to make sure the non-tumor regions were excluded from analysis. The SUV from both cameras were validated and correlated with phantom studies.
SUV was calculated using the following formula:
The SUVmax was obtained by selecting volumetric regions of interest (VOIs) within the primary cancer site to include all tumor tissue but not any non-tumor tissue with potentially higher SUV than that of the tumor. The glucose concentration was also recorded for each patient before the injection of the 18F-FDG radiotracer in each PET scan.
Data collection
In 325 subjects SUVmax of the primary tumor was obtained from the initial PET reports. PET study interpretation had been independently performed by five experienced nuclear medicine physicians. In 38 subjects SUVmax was calculated from the PET images as it was not reported in the initial PET reports.
Baseline demographic, clinical, and tumor characteristics, treatment, follow-up, and survival data were obtained from the electronic medical record system and institutional tumor registry records.
The histological type was categorized according to the WHO classification system [17].
End-point assessment
The primary outcome measure was the duration of overall survival. It was measured from the date of surgery to the date of death from any cause with surviving patients censored at the time of last contact.
Statistical methods
Variables studied included age, race, gender, preoperative SUVmax, pathological stage, tumor size, tumor laterality, type of surgery, histology subtype, and cytologic grade.
The continuous variables SUVmax, tumor size, and age were examined for normality and skewness. SUV and tumor size needed log transformations (with base 2).
Each variable was analyzed using univariate proportional hazards (Cox) regression analysis. Multivariate proportional hazards (Cox) regression analyses were applied to test the SUVmax’s independency of other prognostic factors for the prediction of overall survival.
In the initial multivariate Cox regression model, all variables that on univariate analysis were found to have a p value of less than 0.10 were included as covariates. SUVmax, tumor size, and age were treated as continuous variables. Variables were retained in the subsequent Cox regression modeling if they met the p value of less than 0.05 in the model. Nonsignificant variables were removed by stepwise backward elimination. Pathological staging was excluded from multivariate analysis due to potential interaction with tumor size.
The continuous variables SUVmax, tumor size, and age were then dichotomized by a median split. Survival curves stratified by median SUVmax were estimated by the Kaplan-Meier method and statistical differences were assessed using the log-rank test. Multivariate analyses were repeated after replacing continuous variables with median SUVmax, median tumor size, and median age.
Receiver-operating characteristic (ROC) curves were plotted to find out the optimal cutoff values of SUVmax yielding the maximal sensitivity plus specificity for predicting the overall survival. Survival curves stratified by optimal cutoff SUVmax were estimated by the Kaplan-Meier method, and statistical differences were assessed using the log-rank test. Multivariate analyses were performed again after replacing median SUVmax with optimal cutoff SUVmax.
The data were then stratified according to the pathological stage. The median SUVmax for each specific stage was calculated. For specific stages, survival curves stratified by median SUVmax were estimated by the Kaplan-Meier method, and statistical differences were assessed using the log-rank test. By plotting the ROC curves, we attempted to find out the optimal cutoff values of SUVmax for specific stages yielding the maximal sensitivity plus specificity for predicting the overall survival, but none could be established. No stage-specific analysis was performed for stage IIA due to the small number of subjects.
Statistical analyses were performed using SPSS® version 13.0 (SPSS Inc., Chicago, IL, USA).
In order to address the effects of the partial volume effects, the recovery coefficient (RC) was determined, and the SUVmax was corrected using the diameters of the tumor as the following:
where CF = calibration factor and ID = injected dose.
The partial volume corrected SUV (SUVpvc) was given by:
where SUVbkg = background SUV.
Results
A total of 363 subjects met the inclusion and exclusion criteria. The median duration between preoperative 18F-FDG PET and attempted curative resection was 38 days. The median duration of follow-up was 981 days (2.7 years). The clinicopathologic characteristics are summarized in Table 1 along with the mortality information.
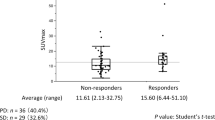
The median SUVmax was 5.9 for all subjects, 4.5 for stage IA, 8.4 for stage IB, and 10.9 for stage IIB (Fig. 1). The optimal cutoff value of SUVmax was 8.2 for all subjects. No optimal cutoff value of SUVmax could be established for specific stages with acceptable sensitivity and specificity for predicting the overall survival. No stage-specific analysis was performed for stage IIA due to the small number of subjects.
In univariate proportional hazards (Cox) regression analysis, each doubling of SUVmax [i.e., each log (base 2) unit increase in SUVmax] was associated with a 1.28-fold [95% confidence interval (CI): 1.0–1.6, p = 0.029] increase in hazard of death (Table 2). Kaplan-Meier survival analyses showed significant difference in overall survival when stratified by median SUVmax (Fig. 2) and optimal cutoff SUVmax (Fig. 3) in the whole group of cases (log-rank test, p = 0.018 and p = 0.004, respectively). The mean survival times of patients with SUVmax of primary tumor equal to or more than median value 5.9 and optimal cutoff value of 8.2 were 66.6 months and 58.9 months, respectively, compared with overall mean survival time of 73.5 months.
Primary analysis with the multivariate Cox proportional hazards model showed that SUVmax was not an independent predictor of overall survival (p > 0.05). Repeat analyses after replacing the continuous variables tumor size, age, and SUVmax with the dichotomous variables median tumor size, median age, and median SUVmax or optimal cutoff SUVmax again failed to show the SUVmax’s independency of other prognostic factors for the prediction of overall survival (p > 0.05 in all multivariate models).
There was high correlation between SUVpvc and the uncorrected SUVmax (Fig. 4). Thus, the statistical analysis on the original data is still valid.
The average size of all tumors was 2.6 ± 1.8 cm (SD). The resolution reported in the method section was at 1 cm. In a realistic clinical setting, the resolution at 10 cm will be a better representation. The full-width half-maximum (FWHM) tangential resolution of PET alone camera was 4.9 mm and the radial resolution was 4.4 mm compared to that of respective resolutions of PET/CT at 5.8 and 6.6 mm. Thus, our average tumor size exceeded twice that of FWHM in both PET cameras.
Subgroup analyses
Univariate proportional hazards (Cox) regression analyses did not show any significant difference in survival by SUVmax when data were stratified according to the pathological stage (p = 0.119, p = 0.818, and p = 0.882 for pTNM stages IA, IB, and IIB, respectively, Table 2).
Kaplan-Meier survival analyses also did not detect any significant survival differences in any of the pathological stage subgroups considered when patients were stratified according to the stage-specific median SUVmax (log-rank test, p = 0.071, p = 0.682, and p = 0.928 for pTNM stages IA, IB, and IIB, respectively, Figs. 5, 6, and 7).
Discussion
Several studies in the past have reported that preoperative SUV is of prognostic value in early-stage (I & II) NSCLC [3–11, 14, 15]. But some recently published data have cast doubt on this conclusion [12, 13]. Hoang et al. [16] also reported similarly disappointing results in advanced-stage (III & IV) NSCLC. The results of the current study are consistent with these recent studies. They confirmed the observation that in early-stage (I & II) NSCLC patients with higher SUV had significantly higher risks of dying; but they also demonstrated that preoperative SUV was not an independent predictor of survival. Some studies have dichotomized age [9, 14]. This can result in significant loss of statistical power [18], which may cause underestimation of the prognostic importance of age and thus can lead to overestimation of the prognostic importance of other variables in the multivariate models. Other studies did not include age in the multivariate models [3, 4]. Tumor size was also not included in the multivariate models in many prior studies [9, 14], which has been an important prognostic factor. In addition, many of them did not control for other potential confounding factors or were limited by small sample size [5–7, 9, 15]. Some studies did not report multivariate analysis results [5, 11, 15]. It is believed that all these factors can at least partly explain the different conclusions reached.
Several studies have tried to establish optimal SUV cutoff values that differentiate between good and poor prognosis groups in NSCLC. Numerous cutoffs have been suggested. These cutoffs have ranged from 4.3 to 10 [3, 4, 6, 7, 9, 10, 12, 13]. But this approach has the risk of artificial reduction in p values and overestimation of prognostic significance [19]. These cutoff points may be data specific and can also introduce a statistical artifact known as the Will Rogers phenomenon [20]. This has made comparison between different studies difficult. Prior studies have tried dichotomization by median split [8, 14, 15], which can result in significant loss of statistical power and therefore might not be suitable for estimation of prognosis [18]. A few authors have tried grouping subjects into more than two groups [5, 11]. The present study analyzed SUVmax as a continuous variable in early-stage (I & II) NSCLC. This prevented the introduction of all the biases associated with dichotomization and results in maximal statistical power. Another strength of the current study was the exclusion of patients who had received any adjuvant or neoadjuvant chemotherapy or radiation therapy. This avoided the profound confounding effect of multiple treatment protocols. Also the current study population was one of the largest reported to date allowing statistical analyses for adjustment for potential confounders. Moreover, to facilitate comparison with prior studies, additional analyses after dichotomization of SUVmax at both optimal cutoff value and median SUVmax were performed. All three methods produced similar results. Therefore, we believe that our results are valid and generalizable. Although no sharp natural binary cutoff likely exists, the higher the preoperative SUVmax, the higher the probability of death.
Subgroup analyses
Recently Hannin et al. [15] reported that in stage I high SUVmax was associated with significantly decreased overall survival. In this study stage I was not subclassified into stages IA and IB. The current study demonstrated that SUVmax would lose its prognostic value after stratification according to pathological staging into IA, IB, and IIB. The current results are consistent with those reported by Downey et al. [13]. In their study prediction of survival by SUVmax was not found to be independent of pathological staging in early-stage NSCLC. In fact, in our study when pTNM stage subgroups IA and IB were combined, univariate proportional hazards (Cox) regression analysis and Kaplan-Meier survival analysis did detect significant survival differences. Thus, the findings of Hannin et al. may be due to the combining of stages IA and IB cancers. Cerfolio et al. [10] also reported a very significant (p < 0.001) difference in overall survival when all patients were stratified by a SUVmax of 10. But this p value rose dramatically in stage-specific analysis when patients were stratified by stage-specific median SUVmax values (p not significant, p = 0.048, and p = 0.028 for stages IA, IB, and II, respectively; no separate p values for stages IIA and IIB were reported). Many previous studies have used the median or optimal cutoff SUV of the combined sample for the stage-specific analysis [6, 13]. As higher stage NSCLC tumors have higher SUV [10], pathological stage distribution of the study population significantly affects the calculated median and optimal cutoff SUV of the combined sample. This has made comparison between different studies difficult. Also one SUV cutoff might not be suitable for all stages. This issue was addressed by using stage-specific median SUVmax for stage-specific analysis. This yielded more generalizable results. The study also showed that there was no stage-specific optimal cutoff value for pTNM stages IA, IB, or IIB with acceptable sensitivity and specificity for predicting the overall survival. This strengthens the conclusion that SUVmax loses its prognostic value after stratification according to pathological staging.
Limitations
One limitation of the study was the retrospective nature of the data. Also the relatively small number of stage II patients somewhat limited the types of statistical analyses possible. This has been a recurrent problem for studies which assessed the outcome of surgically treated early lung cancer patients [6, 9, 13]. Despite these limitations, the current study provides important insights into the prognostic importance of preoperative SUVmax. However, the results were complementary to a previous publication, which was one of the first investigations to declare the absence of relation between SUV and prognosis in 178 patients [21]. Until more data are available to determine this conclusively, it is prudent to avoid making any treatment decisions solely on the basis of preoperative SUVmax without considering other tumor and patient characteristics. Ultimately, a prospective study with even a larger sample size than the current study is needed to conduct stage-specific analyses.
Conclusion
The results demonstrate that each doubling of SUVmax as determined by preoperative PET is associated with a 1.28-fold increase in hazard of death in early-stage (I & II) NSCLC. Preoperative SUVmax is not an independent predictor of overall survival in that it loses its prognostic value in multivariate analyses and also after stratification according to pathological staging.
Abbreviations
- 18F-FDG:
-
18F-Fluoro-2-deoxyglucose
- NSCLC:
-
Non-small cell lung cancer
- PET:
-
Positron emission tomography
- SUVmax :
-
Maximum standardized uptake value
References
Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343(4):254–61.
Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123(1 Suppl):137S–46.
Ahuja V, Coleman RE, Herndon J, Patz EF Jr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer 1998;83(5):918–24.
Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, Verbeken EK, Deneffe GJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 1999;17(10):3201–6.
Dhital K, Saunders CA, Seed PT, O’Doherty MJ, Dussek J. [(18)F]Fluorodeoxyglucose positron emission tomography and its prognostic value in lung cancer. Eur J Cardiothorac Surg 2000;18(4):425–8.
Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002;43(1):39–45.
Jeong HJ, Min JJ, Park JM, Chung JK, Kim BT, Jeong JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun 2002;23(9):865–70.
Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004;22(16):3255–60.
Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005;23(6):1136–43.
Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 2005;130(1):151–9.
Davies A, Tan C, Paschalides C, Barrington SF, O’Doherty M, Utley M, et al. FDG-PET maximum standardised uptake value is associated with variation in survival: analysis of 498 lung cancer patients. Lung Cancer 2007;55(1):75–8.
Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, Hawes SE, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 2007;13(11):3255–63.
Downey RJ, Akhurst T, Gonen M, Park B, Rusch V. Fluorine-18 fluorodeoxyglucose positron emission tomographic maximal standardized uptake value predicts survival independent of clinical but not pathologic TNM staging of resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2007;133(6):1419–27.
Goodgame B, Pillot GA, Yang Z, Shriki J, Meyers BF, Zoole J, et al. Prognostic value of preoperative positron emission tomography in resected stage I non-small cell lung cancer. J Thorac Oncol 2008;3(2):130–4.
Hanin FX, Lonneux M, Cornet J, Noirhomme P, Coulon C, Distexhe J, et al. Prognostic value of FDG uptake in early stage non-small cell lung cancer. Eur J Cardiothorac Surg 2008;33(5):819–23.
Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE 2nd, Patz EF Jr. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol 2008;26(9):1459–64.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (editors). World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. p. 9–124.
Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332(7549):1080.
Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86(11):829–35.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312(25):1604–8.
Vesselle H, Turcotte E, Wiens L, Schmidt R, Takasugi JE, Lalani T, et al. Relationship between non-small cell lung cancer fluorodeoxyglucose uptake at positron emission tomography and surgical stage with relevance to patient prognosis. Clin Cancer Res 2004;10(14):4709–16.
Funding information:
The authors received no funding for this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Agarwal, M., Brahmanday, G., Bajaj, S.K. et al. Revisiting the prognostic value of preoperative 18F-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) in early-stage (I & II) non-small cell lung cancers (NSCLC). Eur J Nucl Med Mol Imaging 37, 691–698 (2010). https://doi.org/10.1007/s00259-009-1291-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1291-x