Abstract
Purpose
Myocardial extractions of mitochondria complex I (MC-I) inhibitors were high and well correlated with flow. This study assessed the potential of MC-I inhibitors to be developed as myocardial perfusion imaging (MPI) agents.
Methods
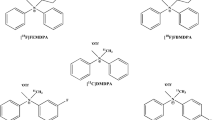
RP1003, RP1004, and RP1005 representing three classes of MC-I inhibitor were synthesized and radio-labeled with 18F. These agents were evaluated for IC50 values, tissue biodistribution, and cardiac PET imaging. 18F-RP1004 was further examined for first-pass extraction and by imaging in non-human primates (NHP) and rats following coronary ligation.
Results
RP1003, RP1004, and RP1005 exhibited high MC-I inhibitory activity with IC50 of 3.7, 16.7, and 14.4 nM. Heart uptakes in rats (percent injected dose per gram tissue) at 15 and 60 min after injection were 3.52 ± 0.36 and 2.68 ± 0.20 for 18F-RP1003, 2.40 ± 0.21 and 2.67 ± 0.27 for 18F-RP1004, and 2.28 ± 0.12 and 1.81 ± 0.17 for 18F-RP1005. The heart to lung and liver uptake ratios were favorable for cardiac imaging with these agents. In isolated perfused rabbit hearts, the uptake of 18F-RP1004 increased from 0.74 ± 0.19 to 1.68 ± 0.39 mL/min/g at flow rates of 1.66 to 5.06 mL/min/g. These values were higher than or similar to that of 99mTc-sestamibi. Cardiac imaging with these agents in rats and rabbits allowed visualization of the heart with minimal lung interference and rapid liver activity clearance. Imaging with 18F-RP1004 also showed clear myocardium and marked liver activity washout in the NHP and clear detection of the perfusion-deficit area associated with left coronary artery ligation in the rat.
Conclusion
MC-I inhibitors have the potential to be a new class of MPI agent.









Similar content being viewed by others
References
Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol 2004;11:71–86.
Garcia EV, Eisner RL, Patterson RE. What should we expect from cardiac PET? J Nucl Med 1993;34:978–80.
Schwaiger M, Melin J. Cardiological applications of nuclear medicine. Lancet 1999;354:661–6.
Lin JW, Sciacca RR, Chou RL, Laine AF, Bergmann SR. Quantification of myocardial perfusion in human subjects using 82Rb and wavelet-based noise reduction. J Nucl Med 2001;42:201–8.
Yu M, Guaraldi MT, Mistry M, Kagan M, McDonald JL, Drew K, et al. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol 2007;14:789–98.
Marshall RC, Powers-Risius P, Reutter BW, O’Neil JP, La Belle M, Huesman RH, et al. Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial flow tracer: comparison to 201Tl. J Nucl Med 2004;45:1950–9.
Marshall RC, Powers-Risius P, Reutter BW, Taylor SE, VanBrocklin HF, Huesman RH, et al. Kinetic analysis of 125I-iodorotenone as a deposited myocardial flow tracer: comparison with 99mTc-sestamibi. J Nucl Med 2001;42:272–81.
Okun JG, Lummen P, Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:Ubiquinone Oxidoreductase). J Biol Chem 1999;274:2625–30.
Schuler F, Yano T, Di Bernardo S, Yagi T, Yankovskaya V, Singer TP, et al. NADH-Quinone oxidoreductase: PSST subunit couples electron transfer from iron-sulfur cluster N2 to quinone. Proc Natl Acad Sci U S A 1999;96:4149–53.
Lindell SD, Ort O, Lummen P, Klein R. The design and synthesis of novel inhibitors of NADH:ubiquinone oxidoreductase. Bioorg Med Chem Lett 2004;14:511–4.
Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 1992;24:669–81.
Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol 2007;14:782–8.
Satoh T, Miyoshi H, Sakamoto K, Iwamura H. Comparison of the inhibitory action of synthetic capsaicin analogues with various NADH-ubiquinone oxidoreductases. Biochim Biophys Acta 1996;1273:21–30.
VanBrocklin HF, Hanrahan SM, Enas JD, Nandanan E, O’Neil JP. Mitochondrial avid radioprobes. Preparation and evaluation of 7’(Z)-[125I]iodorotenone and 7’(Z)-[125I]iodorotenol. Nucl Med Biol 2007;34:109–16.
Piwnica-Worms D, Kronauge JF, Chiu ML. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium(I) in cultured chick myocardial cells. Mitochondrial and plasma membrane potential dependence. Circulation 1990;82:1826–38.
Younes A, Songadele JA, Maublant J, Platts E, Pickett R, Veyre A. Mechanism of uptake of technetium-tetrofosmin. II: Uptake into isolated adult rat heart mitochondria. J Nucl Cardiol 1995;2:327–33.
Glover DK, Ruiz M, Yang JY, Smith WH, Watson DD, Beller GA. Myocardial 99mTc-tetrofosmin uptake during adenosine-induced vasodilatation with either a critical or mild coronary stenosis: comparison with 201Tl and regional myocardial blood flow. Circulation 1997;96:2332–8.
Glover DK, Ruiz M, Edwards NC, Cunningham M, Simanis JP, Smith WH, et al. Comparison between 201Tl and 99mTc sestamibi uptake during adenosine-induced vasodilation as a function of coronary stenosis severity. Circulation 1995;91:813–20.
Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med 2008;49:630–6.
Nekolla SG, Reder S, Higuchi T, Dzewas G, Poethko T, Preissl A, et al. Assessment of imaging properties of a new F-18 labelled flow tracer in a pig model. J Am Coll Cardiol 2008;51:A170.
Hilton TC, Thompson RC, Williams HJ, Saylors R, Fulmer H, Stowers SA. Technetium-99m sestamibi myocardial perfusion imaging in the emergency room evaluation of chest pain. J Am Coll Cardiol 1994;23:1016–22.
Matsunari I, Tanishima Y, Taki J, Ono K, Nishide H, Fujino S, et al. Early and delayed technetium-99m-tetrofosmin myocardial SPECT compared in normal volunteers. J Nucl Med 1996;37:1622–6.
Boz A, Gungor F, Karayalcin B, Yildiz A. The effects of solid food in prevention of intestinal activity in Tc-99m tetrofosmin myocardial perfusion scintigraphy. J Nucl Cardiol 2003;10:161–7.
Hurwitz GA, Clark EM, Slomka PJ, Siddiq SK. Investigation of measures to reduce interfering abdominal activity on rest myocardial images with Tc-99m sestamibi. Clin Nucl Med 1993;18:735–41.
Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 1998;19:26–37.
Acknowledgments
We thank the Veterinary Science Group of Bristol Myers Squibb Medical Imaging for providing excellent animal care. Some of the data were presented at the American Heart Association Scientific Session in 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, M., Guaraldi, M., Kagan, M. et al. Assessment of 18F-labeled mitochondrial complex I inhibitors as PET myocardial perfusion imaging agents in rats, rabbits, and primates. Eur J Nucl Med Mol Imaging 36, 63–72 (2009). https://doi.org/10.1007/s00259-008-0909-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0909-8