Abstract
Purpose
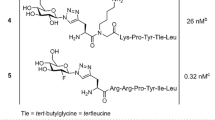
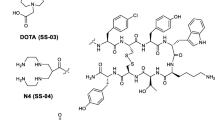
Neurotensin (NT) and its high affinity receptor (NTR1) are involved in several neoplastic processes. Thus, NT-based radiopharmaceuticals are potential tracers for targeted diagnosis and therapy of NTR-positive tumours. A new analogue based on NT(8–13), NT-XIX, with the three enzymatic cleavage sites stabilised, was synthesised and tested.
Methods
The synthesis was performed by Boc strategy. Labelling with 99mTc/188Re was performed using the tricarbonyl technique. Metabolic stability was tested in vitro and in vivo. NT-XIX was further characterised in vitro in HT-29 cells and in vivo in nude mice with HT-29 xenografts.
Results
NT-XIX showed much longer half-lives than non-stabilised analogues. Binding to NTR1 was highly specific, although the affinity was lower than that of natural NT. Bound activity rapidly internalised into HT-29 cells and 50% remained trapped after 24 h. In the time-course biodistribution, the highest uptake was found in the tumour at all p.i. times. In vivo uptake was specific, and accumulation of activity in the kidneys was low. Radioactivity clearance from healthy organs was faster than that from the tumour, resulting in improved tumour-to-tissue ratios and good SPECT/CT imaging. Treatment with 188Re-NT-XIX (30 MBq, in three or four fractions) decreased tumour growth by 50% after 3 weeks.
Conclusion
The high in vivo stability and the favourable in vivo behaviour makes NT-XIX an excellent candidate for the imaging and therapy of NTR1-positive tumours.






Similar content being viewed by others
References
Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–82.
Anderson CJ, Dehdashti F, Cutler D, Schwarz SW, Laforest R, Bass LA, et al. 64Cu-TETA-Octreotide as PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–21.
Evers BM. Neurotensin and growth in normal and neoplastic tissues. Peptides. 2006;27:2424–33.
Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006;27:2445–60.
Carraway RE, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–61.
Nemeroff CB, Luttinger D, Prange AJJ. Neurotensin: central nervous system effects of a neuropeptide. Trends Neurosc. 1980;3:212–5.
Kitabgi P. Effects of neurotensin on intestinal smooth muscle: application to the study of structure–activity relationships. Ann NY Acad Sci. 1982;400:37–55.
Vincent JP, Mazella P, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999;20:302–9.
Mazella J, Zsürger N, Navarro V, Chabry J, Kaghad M, Caput D, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–6.
Morinville A, Martin S, Lavallée M, Vincent JP, Beaudet A, Mazella J. Internalization and trafficking of neurotensin via NTS3 receptors in HT29 cells. Int J Biochem Cell Biol. 2004;36:2153–68.
Reubi JC, Waser B, Friess H, Büchler M, Laissue J. Neurotensin receptors: a new marker for human ductal pancreatic adenocarcinoma. Gut. 1998;42:546–50.
Ehlers RA, Kim S, Zhang Y, Ethridge RT, Murrilo C, Hellmich MR, et al. Gut peptide receptor expression in human pancreatic cancers. Ann Surg. 2000;231:838–48.
Souazé F, Dupouy S, Viardot-Foucault V, Bruyneel E, Attaub S, Gespach C, et al. Expression of neurotensin and NT1 receptor in human breast cancer: a potential role in tumor progression. Cancer Res. 2006;66:6243–9.
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30.
American Cancer Society. Breast cancer facts & figures 2005–2006. Atlanta: American Cancer Society Inc.
Kitabgi P, De Nadai F, Rovère C, Bidard JN. Biosynthesis, maturation, release and degradation of neurotensin and neuromedin N. Ann NY Acad Sci. 1992;668:30–42.
Garcia-Garayoa E, Blaeuenstein P, Bruehlmeier M, Blanc A, Iterbeke K, Conrath P, et al. Preclinical evaluation of a new, stabilized neurotensin(8–13) pseudopeptide radiolabeled with 99mTc. J Nucl Med. 2002;43:374–83.
Bruehlmeier M, Garcia Garayoa E, Blanc A, Holzer B, Gergely S, Tourwé D, et al. Stabilization of neurotensin analogues: effect on peptide catabolism, biodistribution and tumor binding. Nucl Med Biol. 2002;29:321–7.
García Garayoa E, Maes V, Bläuenstein P, Blanc A, Hohn A, Tourwé D, et al. Double-stabilized neurotensin analogues as potential radiopharmaceuticals for NT receptor-positive tumors. Nucl Med Biol. 2006;33:495–503.
Buchegger F, Bonvin F, Kosinski M, Schaffland AO, Prior J, Reubi JC, et al. Radiolabeled neurotensin analog, 99mTc-NT-XI, evaluated in ductal pancreatic adenocarcinoma patients. J Nucl Med. 2003;44:1649–54.
Maes V, García Garayoa E, Bläuenstein P, Tourwé DA. Novel 99mTc-labelled neurotensin analogs with optimized biodistribution properties. J Med Chem. 2006;49:1833–6.
Schibli R, La Bella R, Alberto R, Garcia Garayoa E, Ortner K, Abram U, et al. Influence of the denticity of ligand systems on the in vitro and in vivo behavior of 99mTc(I)-tricarbonyl complexes: a hint for the future functionalization of biomolecules. Bioconjugate Chem. 2000;11:345–51.
García Garayoa E, Allemann-Tannahill L, Bläuenstein P, Willmann M, Carrel-Rémy N, Tourwé D, et al. In vitro and in vivo evaluation of new radiolabeled neurotensin(8–13) analogues with high affinity for NT1 receptors. Nucl Med Biol. 2001;28:75–84.
Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, et al. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317:139–42.
Mazella J, Vincent JP. Internalization and recycling properties of neurotensin receptors. Peptides. 2006;27:2488–92.
de Visser M, Janssen PJJM, Srinivasan A, Reubi JC, Waser B, Erion JL, et al. Stabilised 111In-labelled DTPA- and DOTA-conjugated neurotensin analogues for imaging and therapy of exocrine pancreatic cancer. Eur J Nucl Med Mol Imaging. 2003;30:1134–9.
Achilefu S, Srinivasan A, Schmidt MA, Jimenez HN, Bugaj JE, Erion JL. Novel bioactive and stable neurotensin peptide analogues capable of delivering radiopharmaceuticals and molecular beacons to tumors. J Med Chem. 2003;46:3403–11.
Behr TM, Jenner N, Béhé M, Angerstein C, Gratz S, Raue F, et al. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J Nucl Med. 1999;40:1029–44.
Boerman OC, Oyen WJG, Corstens FHM. Between the Scylla and Charybdis of peptide radionuclide therapy: hitting the tumor and saving the kidney. Eur J Nucl Med. 2001;28:1447–9.
Zhang K, An R, Gao Z, Zhang Y, Aruva MR. Radionuclide imaging of small-cell lung cancer (SCLC) using 99mTc-labeled neurotensin peptide 8–13. Nucl Med Biol. 2006;28:505–12.
Lambert B, Cybulla M, Weiner SM, Van de Wiele C, Ham H, Dierckx RA, et al. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161:607–11.
Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabelled antibody fragments, and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–12.
Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imag. 2003;30:9–15.
Vegt E, Wetzels JFM, Russel FGM, Masereeuw R, Boerman OC, van Eerd JE, et al. Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med. 2006;47:432–6.
Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67:9501–6.
Boerma M, Wang J, Richter KK, Hauer-Jensen M. Orazipone, a locally acting immunomodulator, ameliorates intestinal radiation injury: a preclinical study in a novel rat model. Int J Radiat Oncol Biol Phys. 2006;66:552–9.
Wang J, Hauer-Jensen M. Radiation toxicity and proteinase–activated receptors. Drug Dev Res. 2003;60:1–8.
Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemostasis. 2004;2:2027–35.
Wang J, Boerma M, Fu Q, Kulkarni A, Fink LM, Hauer-Jensen M. Simvastatin ameliorates radiation enteropathy development after localized, fractionated irradiation by a protein C-independent mechanism. Int J Radiat Oncol Biol Phys. 2007;68:1483–90.
Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–47.
Acknowledgements
We thank the Fund for Scientific Research-Flanders Belgium (contract No. G.0036.04) for financial support, Ms. Harriet Struthers for her assistance with editing the manuscript and Ms. Margaretha Lutz for her technical help.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partly funded by the Fund for Scientific Research-Flanders (Belgium), contract No. G.0036.04.
Rights and permissions
About this article
Cite this article
García-Garayoa, E., Bläuenstein, P., Blanc, A. et al. A stable neurotensin-based radiopharmaceutical for targeted imaging and therapy of neurotensin receptor-positive tumours. Eur J Nucl Med Mol Imaging 36, 37–47 (2009). https://doi.org/10.1007/s00259-008-0894-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0894-y