Abstract
Purpose
To compare left ventricular (LV) dyssynchrony assessment by phase analysis from gated myocardial perfusion SPECT (GMPS) with LV dyssynchrony assessment by tri-plane tissue Doppler imaging (TDI). Baseline LV dyssynchrony assessed with standard deviation (SD) of time-to-peak systolic velocity of 12 LV segments (Ts-SD) with TDI has proven to be a powerful predictor of response to CRT. Information on LV dyssynchrony can also be provided by GMPS with phase analysis of regional LV maximal count changes throughout the cardiac cycle.
Methods
Forty heart failure patients, referred for evaluation of potential eligibility for CRT, underwent both 3D echocardiography, with tri-plane TDI, and resting GMPS. From tri-plane TDI, Ts-SD was used as a validated parameter of LV dyssynchrony and compared with different indices (histogram bandwidth, phase SD, histogram skewness and kurtosis) derived from phase analysis of GMPS.
Results
Histogram bandwidth and phase SD showed good correlation with Ts-SD (r=0.77 and r=0.74, p<0.0001, respectively). Patients with substantial LV dyssynchrony assessed with tri-plane TDI (Ts-SD ≥33 ms) had also significantly higher values of histogram bandwidth and phase SD.
Conclusions
The results of this study support the use of phase analysis by GMPS to evaluate LV dyssynchrony. Histogram bandwidth and phase SD showed the best correlation with Ts-SD assessed with tri-plane TDI and appeared the most optimal variables for assessment of LV dyssynchrony with GMPS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac resynchronization therapy (CRT) has become an established therapy for patients with end-stage heart failure [1]. However, up to 30% of the patients do not respond to CRT when selection is based on the traditional clinical and electrocardiographic criteria [New York Heart Association (NYHA) functional class III or IV, left ventricular ejection fraction (LVEF) <35%, and QRS duration >120 ms] [2]. In the search for better selection criteria for CRT, it has been shown that patients with LV dyssynchrony have a higher likelihood of a positive response to CRT [3–8]. Over the past few years, the assessment of LV dyssynchrony has been studied extensively with echocardiography.
The most frequently used technique is tissue Doppler imaging (TDI), which permits evaluation of timing of regional myocardial velocity and comparison of different regions yields information on LV dyssynchrony.
Bax et al. [3] have used a four-segment model to assess LV dyssynchrony (four basal LV segments: anterior, lateral, inferior and septal) and reported that a delay ≥ 65 ms among the four segments was a strong predictor of response to CRT. Other authors extended the analysis by evaluating the activation pattern of the entire LV. This method, originally described by Yu and co-workers [6–8], uses the standard deviation (SD) of electromechanical activation times based on a 12-segment model of LV (Ts-SD). The authors investigated 56 patients with heart failure who underwent CRT implantation and demonstrated that a cut-off value of 34.4 ms for Ts-SD could predict reverse LV remodeling after CRT with a sensitivity of 87% and a specificity of 81% [7]. However, to calculate this parameter of LV dyssynchrony, three apical views need to be acquired separately. A 3-dimensional (3D) TDI imaging modality (tri-plane TDI) has become available to overcome this limitation. Tri-plane TDI allows simultaneous acquisition of all LV segments during the same heartbeat rendering the technique more precise than the 2-dimensional (2D) TDI equivalent. Another recent development is the use of gated myocardial perfusion single photon emission computed tomography (GMPS) for assessment of LV dyssynchrony [9, 10]. A count-based method has been developed to extract phase information from the regional LV count changes throughout the cardiac cycle, and this phase information is related to the time interval when a region, in the 3D LV wall starts to contract (phase analysis). This technique has recently been implemented in the Emory Cardiac Toolbox as a diagnostic tool for assessment of LV mechanical dyssynchrony [9].
To further validate the use of GMPS with phase analysis for the assessment of LV dyssynchrony, a direct comparison with tri-plane TDI was performed in a cohort of heart failure patients.
Materials and methods
Patients and study protocol
Forty consecutive patients with heart failure, referred for evaluation of potential eligibility for CRT, were included in this study. Traditional selection criteria for CRT were applied, including moderate-to-severe drug refractory heart failure (NYHA class III or IV), depressed LVEF (<35%) and prolonged QRS duration (>120 ms). Patients with a recent myocardial infarction (<3 months) or decompensated heart failure were excluded. Evaluation of the clinical status included assessment of NYHA functional class, quality-of-life score (using the Minnesota quality-of-life questionnaire) and exercise capacity (using the 6-min walk test). Extensive echocardiographic analysis, using 3D transthoracic echocardiography, was performed to assess LV dyssynchrony. Resting GMPS with technetium-99m tetrofosmin was clinically performed to exclude extensive ischemia and/or viability [11, 12], in order to refer patients to revascularization if indicated. Thereafter, all scans were subjected to phase analysis to evaluate LV dyssynchrony. The results of the phase analysis with GMPS to assess LV dyssynchrony were subsequently compared with LV dyssynchrony derived from tri-plane TDI.
Echocardiography
3D data set acquisition
All patients underwent transthoracic echocardiography performed with a commercially available echocardiographic platform (VIVID 7, GE Vingmed Ultrasound, Horten, Norway) and equipped with a 3V-probe for 3D acquisition.
Patients were scanned in left lateral decubitus position, from the apical window in tri-plane modus, acquiring simultaneously the apical four-, two- and three-chamber views. Care was taken to visualize the true LV apex. Color-coded TDI was applied to the tri-plane view to assess longitudinal myocardial regional function. Gain settings, filters and pulse repetition frequency were adjusted to optimize color saturation. Sector size and depth were optimized for the highest possible frame rate. At least two consecutive beats were recorded from each view, and the images were digitally stored for off-line analysis (EchoPac, GE Vingmed Ultrasound, Horten, Norway). The echocardiographic examination and the off-line analysis were performed by the same experienced echocardiographist, blinded to the GMPS and clinical data.
3D analysis of LV dyssynchrony
During post-processing, the tri-plane TDI dataset was used to analyze myocardial velocity curves as previously described with 2D TDI echocardiography [6–8]. Sample volumes were placed in the basal and mid segments of the septal, lateral, inferior, anterior, posterior and anteroseptal LV walls to calculate the time from the beginning of the QRS complex to peak systolic velocity (Ts), using the same heart beat (Fig. 1). Subsequently, the standard deviation of Ts (Ts-SD) of the 12 myocardial segments was calculated and used as a parameter of LV dyssynchrony [6–8]. A cut-off value of Ts-SD ≥ 33 ms was used to define the presence of substantial LV dyssynchrony.
Example of the myocardial velocity curves that can be derived by positioning the sample volume in any LV segment of the tri-plane dataset. This patient has substantial LV dyssynchrony: the postero-lateral and anterior walls (orange, light blue and red curves) are activated later than the septal and inferior walls (yellow and green curves); standard deviation of 12 LV segments' Ts is 57.5 ms
Gated myocardial perfusion SPECT
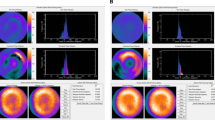
Resting GMPS with technetium-99m tetrofosmin (500 MBq, injected at rest) was performed using a triple head SPECT camera system (GCA 9300/HG, Toshiba Corp.) equipped with low energy high-resolution collimators. Around the 140-KeV energy peak of technetium-99m tetrofosmin, a 20% window was used. A total of 90 projections (step and shoot mode, 35 s per projection, imaging time 23 min) were obtained over a 360-degrees circular orbit. GMPS acquisition involved 16 frames per cardiac cycle. Data were reconstructed by filtered backprojection and then re-oriented to yield gated short-axis images. Data reconstruction was performed over 360 degrees. These images were then submitted to the Emory Cardiac Toolbox for phase analysis [9]. GMPS images were analyzed at the department of radiology of Emory University School of Medicine by J.C. and E.V.G., who were blinded to the echocardiographic and clinical data. A phase distribution was extracted from a GMPS study, representing the regional onset of mechanical contraction of the LV. It can be displayed in a polar map or in 3D and used to generate a phase histogram. An example of a patient without LV dyssynchrony is shown in Fig. 2a, whereas an example of a patient with substantial LV dyssynchrony as assessed with phase analysis by GMPS is shown in Fig. 2b. The following four quantitative indices were obtained from the phase analysis of all patients: (1) histogram bandwidth, which includes 95% of the elements of the phase distribution; (2) phase SD, which is the SD of the phase distribution; (3) phase histogram skewness, which indicates the symmetry of the histogram; (4) histogram kurtosis, which indicates the degree to which the histogram is peaked [9]. In a healthy individual the LV contracts in a coordinated manner, and the phase distribution is nearly uniform with a highly peaked distribution of the histograms. As the LV contraction becomes dyssynchronous, the phase SD and histogram bandwidth are expected to increase (Fig. 2b).
a Example of a patient without LV dyssynchrony on GMPS. The non-normalized (upper panel) and normalized (lower panel) phase distributions are nearly uniform and the corresponding phase histograms are highly-peaked, narrow distributions. b Example of a patient (same as in Fig. 1) with LV dyssynchrony on GMPS. The non-normalized (upper panel) and normalized (lower panel) phase distributions show significant non-uniformity and the corresponding phase histograms have widespread distributions
Statistical analysis
Continuous data are presented as mean ± SD; dichotomous data are presented as numbers and percentages. Comparison of data was performed using the unpaired Student's t test or χ2 test when appropriate. Pearson’s correlation analysis was performed to evaluate the relation between histogram bandwidth, phase SD, histogram skewness and histogram kurtosis by the phase analysis of GMPS and LV dyssynchrony (Ts-SD) by tri-plane TDI. A p-value <0.05 was considered to be statistically significant. A statistical software program SPSS 12.0 (SPSS Inc, Chicago, Il) was used for statistical analysis.
Results
Study population
A total of 40 consecutive patients with heart failure were included in this study [29 (72%) men; mean age 66±10 years]. Baseline characteristics are summarized in Table 1. Patients had severe LV dysfunction (mean LVEF 26 ±7%), with extensive LV dilatation (mean LV end-diastolic volume 210±48 ml). LV dyssynchrony assessment with tri-plane TDI demonstrated a mean value of Ts-SD of 34±15 ms.
Histogram bandwidth
The mean histogram bandwidth was 130°±69° (range 41° to 260°); the normal value is 38.7°±11.8° for men and 30.6°±9.6° for women [9]. A good correlation between histogram bandwidth and LV dyssynchrony, assessed with tri-plane TDI, was observed (r=0.77, p<0.0001, Fig. 3).
Phase SD
The mean phase SD was 40.2°±18.8° (range 14.5° to 77.9°), whereas the normal value is 14.2°±5.1° for men and 11.8°±5.2° for women [9]. Pearson’s correlation showed a good correlation between phase SD and Ts-SD (r=0.74, p<0.0001, Fig. 4).
Histogram skewness
The mean histogram skewness was 2.50±0.81 (range 1.16 to 5.49), whereas the normal value is 4.19±0.68 for men and 4.60±0.72 for women [9]. A poor correlation with LV dyssynchrony assessed with tri-plane TDI was demonstrated by Pearson’s correlation (r=-0.30, p=0.06).
Histogram kurtosis
The mean histogram kurtosis was 7.38±6.23 (range 1.08 to 37.42), whereas the normal value is 19.72±7.68 for men and 23.21±8.16 for women [9]. No correlation between histogram kurtosis and Ts-SD was shown by Pearson’s correlation (r=−0.14, p=0.38).
Substantial versus no substantial LV dyssynchrony
Of the 40 patients included, 20 (50%) had a substantial LV dyssynchrony (defined as Ts-SD ≥ 33 ms) [7, 8]. No significant differences in baseline characteristics were noted between the two patient groups (Table 2). Histogram bandwidth and phase SD were significantly higher in patients with Ts-SD ≥ 33 ms compared to patients with Ts-SD <33 ms: 186°±52° versus 74°±24° (p<0.0001) and 55.3°±13.6° versus 25.1°±7.6° (p<0.0001), respectively (Fig. 5a and b). In addition, histogram skewness was significantly lower in patients with Ts-SD ≥ 33 ms than in patients with Ts-SD <33 ms (2.23±0.93 versus 2.78±0.55, p=0.03). No significant difference between the two groups was observed for histogram kurtosis (6.36±7.94 versus 8.4±3.79, p=NS) (Fig. 5c and d). An example of a patient with substantial LV dyssynchrony on both tri-plane TDI as well as on phase analysis with GMPS is provided in Figs. 1 and 2b.
a Patients with substantial versus no substantial LV dyssynchrony (Ts-SD), using a cut-off value ≥33 ms. Histogram bandwidth is significantly higher in patients with substantial LV dyssynchrony. b Patients with substantial versus no substantial LV dyssynchrony (Ts-SD), using a cut-off value ≥33 ms. Phase SD is significantly higher in patients with substantial LV dyssynchrony. c Patients with substantial versus no substantial LV dyssynchrony (Ts-SD), using a cut-off value ≥33 ms. Histogram skewness is significantly lower in patients with substantial LV dyssynchrony. d Patients with substantial versus no substantial LV dyssynchrony (Ts-SD), using a cut-off value ≥33 ms. No significant difference in histogram kurtosis was demonstrated between patients without and patients with substantial LV dyssynchrony as assessed with tri-plane echocardiography
Ischemic versus idiopathic dilated cardiomyopathy
In total, 25 patients (62%) had an ischemic cardiomyopathy and 15 patients (38%) had an idiopathic dilated cardiomyopathy. No differences in echocardiographic variables, including LV dyssynchrony assessed by tri-plane TDI, or GMPS phase analysis indices were found between ischemic and non-ischemic patients. No significant differences in correlation coefficients were observed between patients with ischemic cardiomyopathy and patients with idiopathic dilated cardiomyopathy for the four different GMPS variables (Table 3).
Discussion
The main findings of the present study can be summarized as follows:
(1) LV dyssynchrony assessment with phase analysis on GMPS is feasible in heart failure patients screened for eligibility for CRT implantation. The phase analysis variables histogram bandwidth and phase SD show good correlation with LV dyssynchrony as measured with tri-plane TDI (Ts-SD), suggesting that GMPS phase analysis can be used to quantify LV dyssynchrony. (2) Patients with substantial LV dyssynchrony on tri-plane TDI have significantly higher values of histogram bandwidth and phase SD derived from phase analysis with GMPS (histogram bandwidth: 186°±52° versus 74°±24°, p<0.0001) and phase SD: 55.3°±13.6° versus 25.1°±7.6° (p<0.0001).
CRT is now considered an important therapeutic option in the treatment of patients with end-stage heart failure [1]. Although encouraging beneficial effects have been reported, including improvement in LVEF and reduction in heart failure symptoms, it has also been demonstrated that up to 30% of patients do not have a favorable response to CRT. The presence of LV dyssynchrony is considered a key issue in the identification of potential responders to CRT [3–6]. Echocardiography and in particular TDI have been extensively used to assess LV dyssynchrony. Several parameters derived from myocardial velocity curves of different LV segments have been proposed to quantify LV dyssynchrony and predict response to CRT [3–8]. Yu and coworkers described a model (derived from 2D TDI data) to quantify global LV dyssynchrony using Ts-SD of 12 LV segments [6–8]. The authors reported a cut-off value >32.6 ms to distinguish between responders and non-responders [8] and a cut-off value >34.4 ms as the most powerful predictor of LV reverse remodeling [7]. In this study LV dyssynchrony was evaluated by Ts-SD from tri-plane TDI and compared with LV dyssynchrony assessment with phase analysis by GMPS. The tri-plane TDI technique avoids the problem of heart rate variability since simultaneous acquisition of the three apical views is possible; in the present study a cut-off value ≥ 33 ms was used to define substantial LV dyssynchrony on tri-plane TDI.
Information on cardiac dyssynchrony can also be derived from nuclear imaging. Kerwin et al. [13] evaluated interventricular dyssynchrony in 13 heart failure patients using multi-gated equilibrium blood pool scintigraphy and phase analysis. Patients were imaged during biventricular pacing and in sinus rhythm. During biventricular pacing, a significant improvement in interventricular synchrony was observed. Moreover, an improvement in LVEF was reported for all 13 patients during biventricular pacing (17.2±7.9% to 22.5±8.3%, p<0.0001), and the increase in LVEF correlated significantly with the improvement in interventricular synchrony (r=0.86; p<0.001). Fauchier et al. [14] used equilibrium radionuclide angiography and phase analysis to evaluate the prognostic value of interventricular and intraventricular dyssynchrony in 103 patients with idiopathic dilated cardiomyopathy. Phase images were generated from the scintigraphic data, and mean phase angles and SDs were calculated for the right and left ventricle. During a follow-up period of 27±23 months, 18 major cardiac events occurred. Multivariate analysis revealed that LV intraventricular dyssynchrony and elevated pulmonary capillary wedge pressure were the only independent predictors of cardiac events.
GMPS is currently used in patients with heart failure to exclude ischemia and/or viability before referring for CRT implantation and to assess total scar burden in patients with ischemic cardiomyopathy [11, 12]. Indeed, various studies have shown that the presence of severe resting perfusion defects, as assessed with GMPS, limits the response to CRT [15–17]. Recently, Chen et al. [9] developed with GMPS a count-based method to extract phase information from the regional LV count changes throughout the cardiac cycle. The phase information is related to the regional onset of mechanical contraction of the LV (phase analysis) and provides information on the synchronicity of the LV contraction. The authors assessed in 90 normal individuals the normal range for four quantitative indices that can be used as markers of LV dyssynchrony (histogram bandwidth, phase SD, histogram skewness and histogram kurtosis) [9]. Recently, these four GMPS indices have been compared with LV dyssynchrony assessment by TDI in 75 patients with severe heart failure. It was shown that among the four quantitative indices of phase analysis, the variables histogram bandwidth and phase SD correlated best with LV dyssynchrony as assessed by TDI [10]. The current study involved a different patient subset and provides further support that phase analysis with GMPS can be useful in the evaluation of LV dyssynchrony.
The value of GMPS to assess LV dyssynchrony has been analyzed, and the indices derived from phase analysis were compared with Ts-SD measured by tri-plane TDI. It was shown that histogram bandwidth and phase SD correlated well with Ts-SD as evaluated by tri-plane TDI (histogram bandwidth: r=0.77 and phase SD: r=0.74). In addition, significantly higher values for histogram bandwidth and phase SD were observed in patients with substantial LV dyssynchrony on tri-plane TDI compared with patients without substantial LV dyssynchrony on tri-plane TDI.
Limitations
In the present study, follow-up data after CRT implantation were not available. Therefore, the value of phase analysis to predict response to CRT could not be determined. Furthermore, GMPS may be less suitable for repeated analysis during the follow-up after CRT implantation, due to the radiation burden of the technique.
Conclusions
The results of this study confirm the feasibility to evaluate LV dyssynchrony with phase analysis by GMPS and its applicability in the clinical setting. In particular, histogram bandwidth and phase SD showed a good correlation with LV dyssynchrony measured with Ts-SD as assessed with tri-plane TDI. Future prospective studies in larger patient populations with follow-up after CRT implantation are needed to elucidate the potential role of GMPS with phase analysis for prediction of response to CRT.
References
Freemantle N, Tharmanathan P, Calvert MJ, Abraham WT, Ghosh J, Cleland JG. Cardiac resynchronisation for patients with heart failure due to left ventricular systolic dysfunction-a systematic review and meta-analysis. Eur J Heart Fail 2006;8:433–40.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53.
Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834–40.
Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO, et al. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol 2002;40:723–30.
Notabartolo D, Merlino JD, Smith AL, Delurgio DB, Vera FV, Easley KA, et al. Usefulness of the peak velocity difference by tissue Doppler imaging technique as an effective predictor of response to cardiac resynchronization therapy. Am J Cardiol 2004;94:817–20.
Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002;105:438–45.
Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol 2005;45:677–84.
Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003;91:684–8.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687–95.
Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion SPECT compared to tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol 2007;49:1708–14.
Levine MG, McGill CC, Ahlberg AW, White MP, Giri S, Shareef B, et al. Functional assessment with electrocardiographic gated single-photon emission computed tomography improves the ability of technetium-99m sestamibi myocardial perfusion imaging to predict myocardial viability in patients undergoing revascularization. Am J Cardiol 1999;83:1–5.
Udelson JE, Coleman PS, Metherall J, Pandian NG, Gomez AR, Griffith JL, et al. Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation 1994;89:2552–61.
Kerwin WF, Botvinick EH, O’Connell JW, Merrick SH, DeMarco T, Chatterjee K, et al. Ventricular contraction abnormalities in dilated cardiomyopathy: effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol 2000;35:1221–7.
Fauchier L, Marie O, Casset-Senon D, Babuty D, Cosnay P, Fauchier JP. Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: a prognostic study with fourier phase analysis of radionuclide angioscintigraphy. J Am Coll Cardiol 2002;40:2022–30.
Sciagra R, Giaccardi M, Porciani MC, Colella A, Michelucci A, Pieragnoli P, et al. Myocardial perfusion imaging using gated SPECT in heart failure patients undergoing cardiac resynchronization therapy. J Nucl Med 2004;45:164–8.
Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J 2007;28:33–41.
Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105–12.
Acknowledgements
This work was financially supported by:
The Netherlands Heart Foundation, The Hague, The Netherlands, grant number 2002B109.
The European Society of Cardiology, Research Fellowship
Conflict of interest statement
J.J. Bax has received research grants from GE Healthcare and BMS Medical Imaging. E.V. Garcia receives royalties from the sale of the Emory Cardiac Toolbox. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest practice. G.B. Bleeker is financially supported by the Netherlands Heart Foundation, grant no. 2002B109. N. Ajmone Marsan is financially supported by the Research Fellowship of the European Society of Cardiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ajmone Marsan, N., Henneman, M.M., Chen, J. et al. Left ventricular dyssynchrony assessed by two three-dimensional imaging modalities: phase analysis of gated myocardial perfusion SPECT and tri-plane tissue Doppler imaging. Eur J Nucl Med Mol Imaging 35, 166–173 (2008). https://doi.org/10.1007/s00259-007-0539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0539-6