Abstract
Purpose
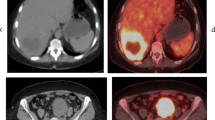
The aim of this study was to assess the performance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) versus dedicated contrast-enhanced CT (CECT) in the detection of metastatic liver disease.
Methods
All patients that presented to our Institution with suspected metastatic liver disease who underwent 18F-FDG PET/CT and CECT within 6 weeks of each other, were retrospectively analyzed, covering a 5-year period. One hundred and thirty-one patients (67 men, 64 women; mean age 62) were identified. Seventy-five had colorectal carcinoma and 56 had other malignancies. The performance of CECT and that of 18F-FDG-PET/CT in detecting liver metastases were compared. The ability of each to detect local recurrence, extrahepatic metastases and to alter patient management was recorded. The final diagnosis was based on histology, clinical and radiological follow-up (mean 23 months).
Results
In detecting hepatic metastases, 18F-FDG-PET/CT yielded 96% sensitivity and 75% specificity, whilst CECT showed 88% sensitivity and 25% specificity. 18F-FDG-PET/CT and CECT were concordant in 102 out of 131 patients (78%). In the colorectal group 18F-FDG-PET/CT showed 94% sensitivity and 75% specificity, whilst CECT had 91% sensitivity and 25% specificity. In the noncolorectal group 18F-FDG-PET/CT showed 98% sensitivity and 75% specificity whilst CECT had 85% sensitivity and 25% specificity. Overall, 18F-FDG-PET/CT altered patient management over CECT in 25% of patients. CECT did not alter patient management over 18F-FDG-PET/CT alone in any patients.
Conclusion
18F-FDG-PET/CT performed better in detecting metastatic liver disease than CECT in both colorectal and noncolorectal malignancies, and frequently altered patient management. The future role of CECT in these patients may need to be re-evaluated to avoid potentially unnecessary duplication of investigation where 18F-PET/CT is readily available.
Similar content being viewed by others

References
Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology 2002;S224:748–56.
Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—meta-analysis. Radiology 2005;237:123–31.
Seemann MD, Meisetschlaeger G, Gaa J, Rummeny EJ. Assessment of the extent of metastases of gastrointestinal carcinoid tumors using whole-body PET, CT, MRI, PET/CT and PET/MRI. Eur J Med Res 2006;11:58–65.
Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg 2004;240:1027–34.
Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK Jr, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg 1998;133:510–6.
Findlay M, Young H, Cunningham D, Iveson A, Cronin B, Hickish T, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 1996;14:700–8.
Selzner M, Clavien PA. Resection of liver tumors: special emphasis on neoadjuvant and adjuvant therapy. In: Clavien PA, editor. Malignant liver tumors: current and emerging therapies. Malden: Blackwell Science; 1999. pp. 137–49.
Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38–46.
Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with vs. without ischemic preconditioning. Ann Surg 2003;238:843–52.
Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 1995;169:36–42.
Ihse I, Persson B, Tibblin S. Neuroendocrine metastases of the liver. World J Surg 1995;19:76–82.
Schwartz SI. Hepatic resection for noncolorectal nonneuroendocrine metastases. World J Surg 1995;19:72–5.
Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery 1997;121:625–32.
Kavolius J, Fong Y, Blumgart LH. Surgical resection of metastatic liver tumors. Surg Oncol Clin North Am 1996;5:337–51.
Nunobe S, Sano T, Shimada K, Sakamoto Y, Kosuge T. Surgery including liver resection for metastatic gastrointestinal stromal tumors or gastrointestinal leiomyosarcomas. Jpn J Clin Oncol 2005;35:338–41.
DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to the liver. Ann Surg 2001;234:540–8.
Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, Plaud B, Ducreux M, Spielmann M, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg 1998;187:487–93.
D’Angelica M, Jarnagin W, Dematteo R, Conlon K, Blumgart LH, Fong Y. Staging laparoscopy for potentially resectable noncolorectal, nonneuroendocrine liver metastases. Ann Surg Oncol 2002;9:204–9.
Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D’Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg 2005;241:269–76.
Frohlich A, Diederichs CG, Staib L, Vogel J, Beger HG, Reske SN. Detection of liver metastases from pancreatic cancer using FDG PET. J Nucl Med 1999;40:250–5.
Celli N, Gaiani S, Piscaglia F, Zironi G, Camaggi V, Leoni S, et al. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol 2007;19(1):3–14.
Schmidt GP, Baur-Melnyk A, Herzog P, Schmid R, Tiling R, Schmidt M, et al. High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography-computed tomography: experience on a 32-channel system. Invest Radiol 2005;40 (12):743–53.
Schmidt GP, Schoenberg SO, Schmid R, Stahl R, Tiling R, Becker C, et al. Screening for bone metastases: whole-body MRI using a 32-channel system versus dual-modality PET-CT. Eur Radiol 2007;17(4):939–49.
Haider MA, Amitai MM, Rappaport DC, O’Malley ME, Hanbidge AE, Redston M, et al. Multi-detector row helical CT in preoperative assessment of small (< or = 1.5 cm) liver metastases: is thinner collimation better? Radiology 2002;225:137–42.
Kim YK, Ko SW, Hwang SB, Kim CS, Yu HC. Detection and characterization of liver metastases: 16-slice multidetector computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur Radiol 2006;16:1337–45.
Onishi H, Murakami T, Kim T, Hori M, Iannaccone R, Kuwabara M, et al. Hepatic metastases: detection with multi-detector row CT, SPIO-enhanced MR imaging, and both techniques combined. Radiology 2006;239:131–8.
Acknowledgements
This work was undertaken at UCLH/UCL who received a proportion of funding from the UK’s Department of Health’s NIHR Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors stated no financial relationship to disclose
Rights and permissions
About this article
Cite this article
Chua, S.C., Groves, A.M., Kayani, I. et al. The impact of 18F-FDG PET/CT in patients with liver metastases. Eur J Nucl Med Mol Imaging 34, 1906–1914 (2007). https://doi.org/10.1007/s00259-007-0518-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0518-y